Содержание
- 2. In the 18th century scientist thought that when things burns a substance call "phlogiston" came out
- 3. Then experiments with closed vessels where substances could be accurately weighed, help scientist such as Antoine
- 4. LAW OF MASS CONSERVATION (1789) In chemical reactions no matter is lost or grained
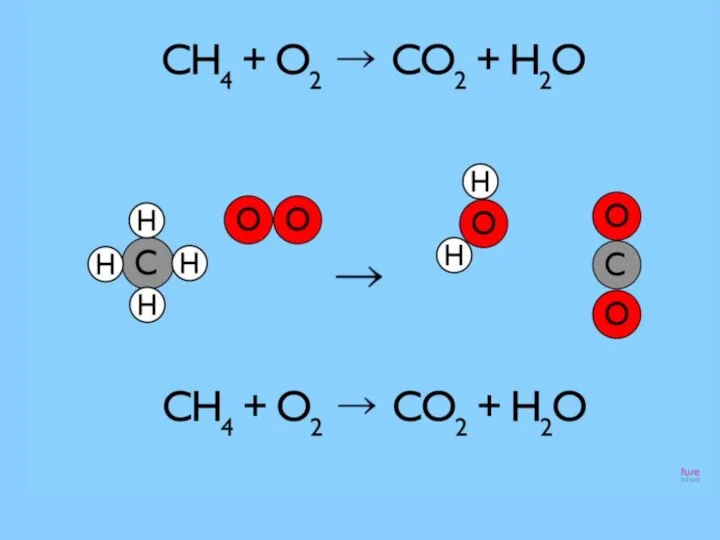
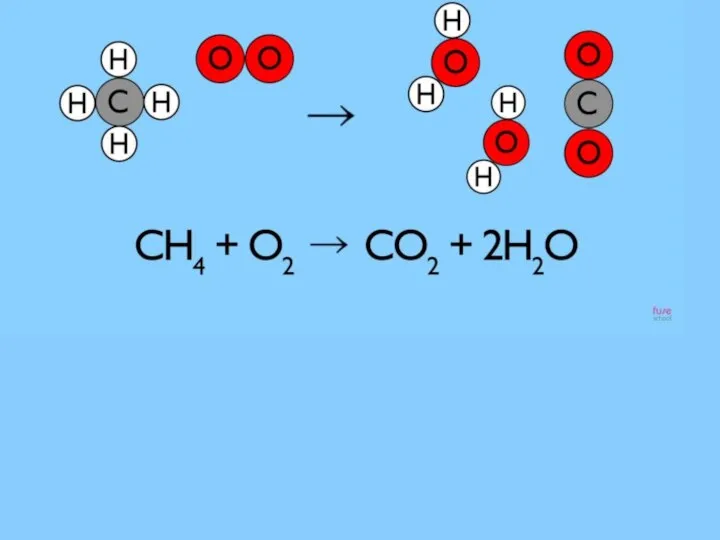
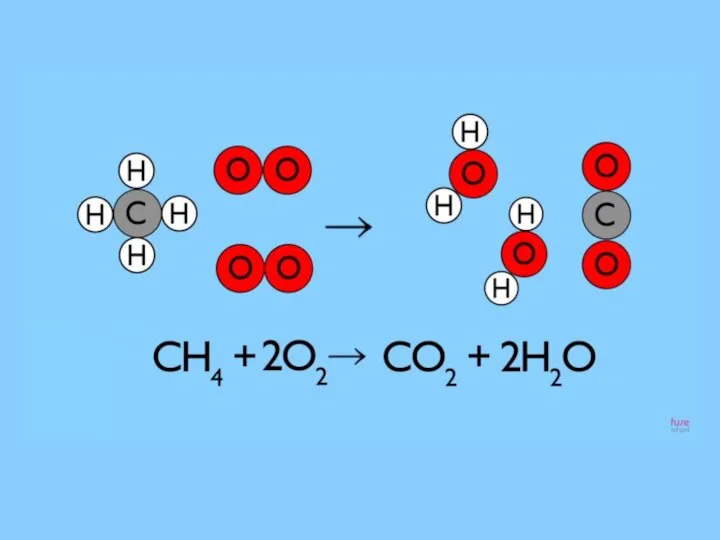
- 5. Reaction that we see when we using the gas cooker which uses natural gas
- 10. Скачать презентацию




 Правила безопасни 7-й класс
Правила безопасни 7-й класс Радиохимия. Альфа-бета, және гамма-сәулеленудің табиғаты және қасиеті
Радиохимия. Альфа-бета, және гамма-сәулеленудің табиғаты және қасиеті Биоэнергетика. Энергетическое сопряжение
Биоэнергетика. Энергетическое сопряжение Типы химических реакций
Типы химических реакций Расчеты по уравнениям химической реакции
Расчеты по уравнениям химической реакции Шкідливий вплив тютюнопаління
Шкідливий вплив тютюнопаління Рефлексия
Рефлексия Коррозионные процессы
Коррозионные процессы Классификация сталей
Классификация сталей Косметические средства. Виды, состав и влияние на организм
Косметические средства. Виды, состав и влияние на организм Молекулы и атомы
Молекулы и атомы Эстетическая, биологическая и культурная роль коллоидных систем в жизни человека
Эстетическая, биологическая и культурная роль коллоидных систем в жизни человека Сложные эфиры. Содержание. Определение
Сложные эфиры. Содержание. Определение Основы кристаллографии
Основы кристаллографии Элемент иттербий
Элемент иттербий Виконала: учениця 11-А класу Сиротенко Катерина Вчитель: Коломійченко Б. Л.
Виконала: учениця 11-А класу Сиротенко Катерина Вчитель: Коломійченко Б. Л.  Синтетические волокна
Синтетические волокна Кристаллические решетки
Кристаллические решетки Основные химические понятия
Основные химические понятия Роль побутової хімії у житті
Роль побутової хімії у житті Синтез наноматериалов на границах раздела жидкость - жидкость и жидкость - воздух. Метод Ленгмюра - Блоджетт
Синтез наноматериалов на границах раздела жидкость - жидкость и жидкость - воздух. Метод Ленгмюра - Блоджетт Чистые вещества и смеси
Чистые вещества и смеси Электроизоляционные пластмассы
Электроизоляционные пластмассы Колообіг нітрогену в природі
Колообіг нітрогену в природі Нефть, её производство, авиационное и дизельное топливо
Нефть, её производство, авиационное и дизельное топливо Метод Кнехта
Метод Кнехта Пластмассы, резины и композиционные материалы
Пластмассы, резины и композиционные материалы Влияние межмолекулярных F…F контактов на энергию кристаллической решетки металлоорганических комплексов Ge
Влияние межмолекулярных F…F контактов на энергию кристаллической решетки металлоорганических комплексов Ge