Содержание
- 2. Overview: Life’s Operating Instructions In 1953, James Watson and Francis Crick introduced an elegant double-helical model
- 3. Figure 16.1
- 4. Concept 16.1: DNA is the genetic material Early in the 20th century, the identification of the
- 5. The Search for the Genetic Material: Scientific Inquiry When T. H. Morgan’s group showed that genes
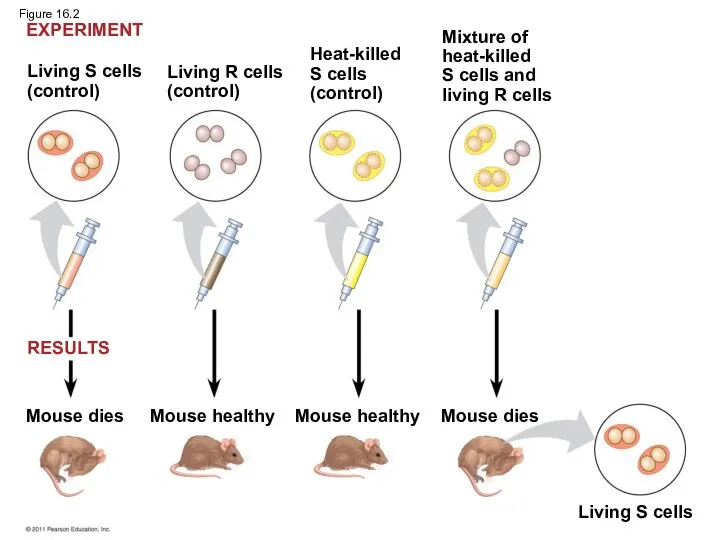
- 6. Evidence That DNA Can Transform Bacteria The discovery of the genetic role of DNA began with
- 7. When he mixed heat-killed remains of the pathogenic strain with living cells of the harmless strain,
- 8. Living S cells (control) Living R cells (control) Heat-killed S cells (control) Mixture of heat-killed S
- 9. In 1944, Oswald Avery, Maclyn McCarty, and Colin MacLeod announced that the transforming substance was DNA
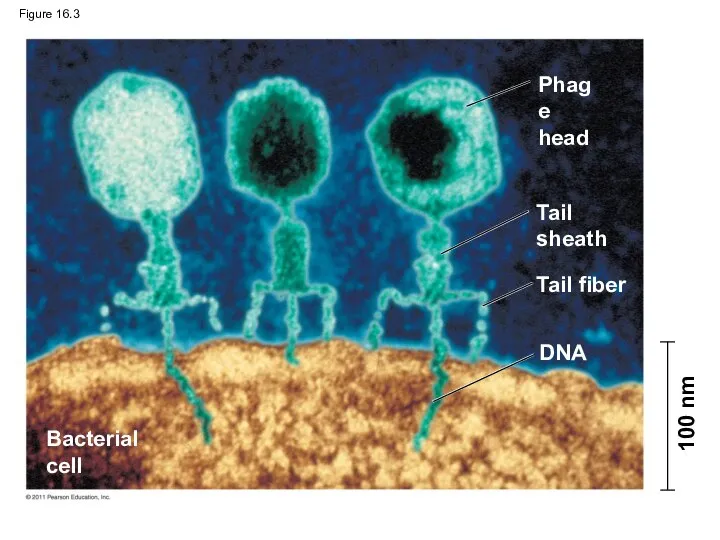
- 10. Evidence That Viral DNA Can Program Cells More evidence for DNA as the genetic material came
- 11. Animation: Phage T2 Reproductive Cycle Right-click slide / select “Play”
- 12. Figure 16.3 Phage head Tail sheath Tail fiber DNA Bacterial cell 100 nm
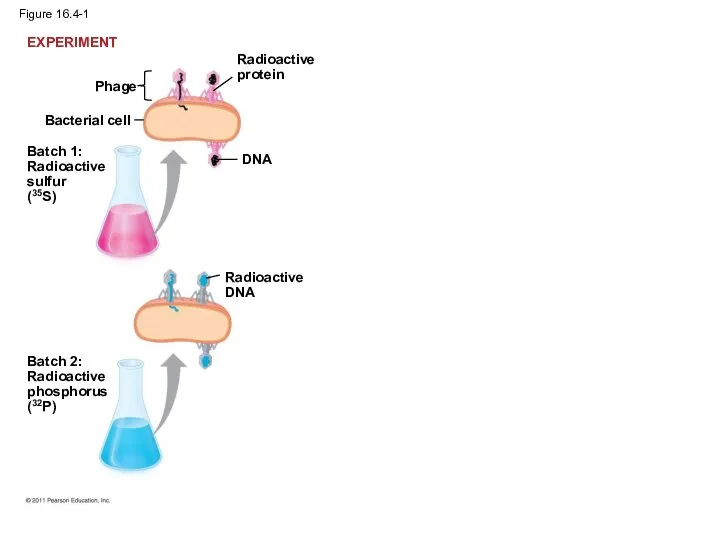
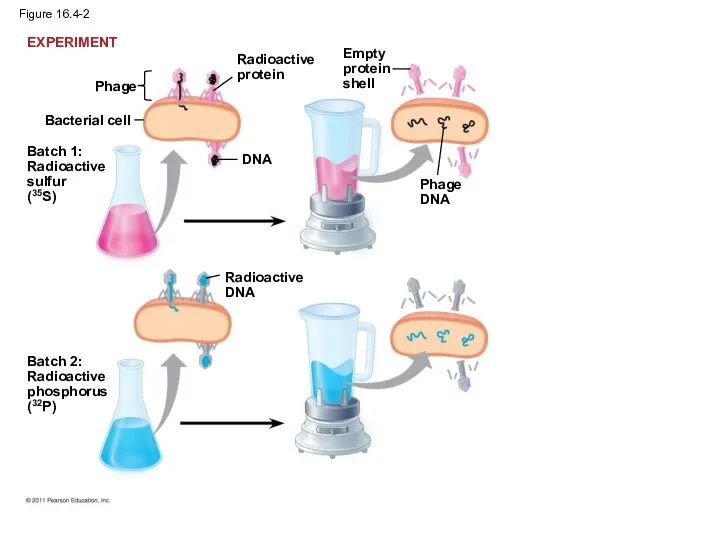
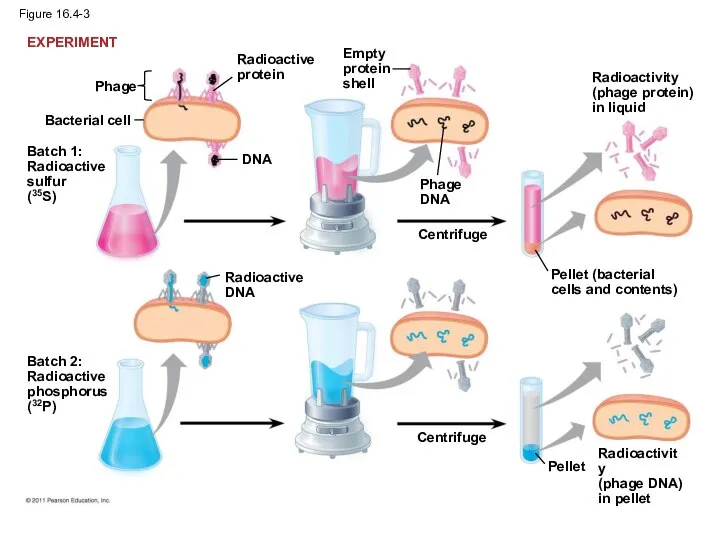
- 13. In 1952, Alfred Hershey and Martha Chase performed experiments showing that DNA is the genetic material
- 14. Animation: Hershey-Chase Experiment Right-click slide / select “Play”
- 15. Figure 16.4-1 Bacterial cell Phage Batch 1: Radioactive sulfur (35S) DNA Batch 2: Radioactive phosphorus (32P)
- 16. Figure 16.4-2 Bacterial cell Phage Batch 1: Radioactive sulfur (35S) Radioactive protein DNA Batch 2: Radioactive
- 17. Figure 16.4-3 Bacterial cell Phage Batch 1: Radioactive sulfur (35S) Radioactive protein DNA Batch 2: Radioactive
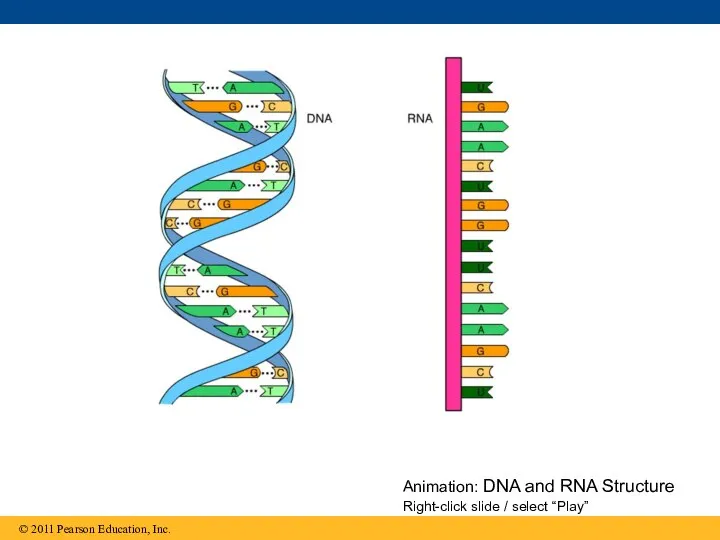
- 18. Additional Evidence That DNA Is the Genetic Material It was known that DNA is a polymer
- 19. Animation: DNA and RNA Structure Right-click slide / select “Play”
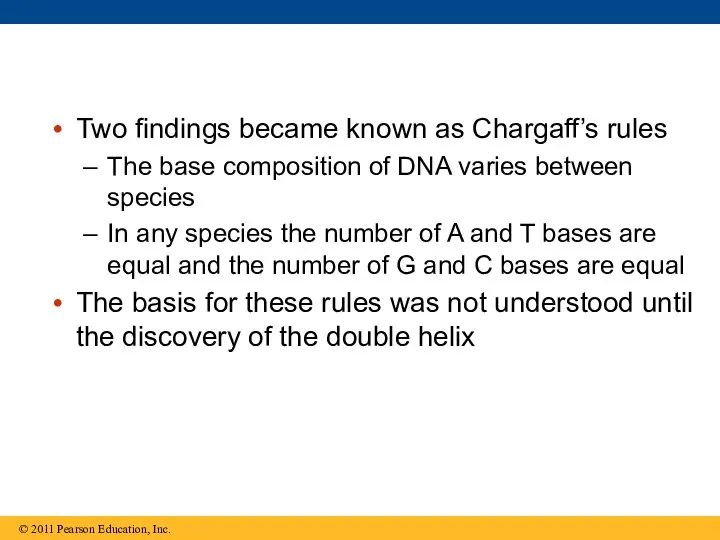
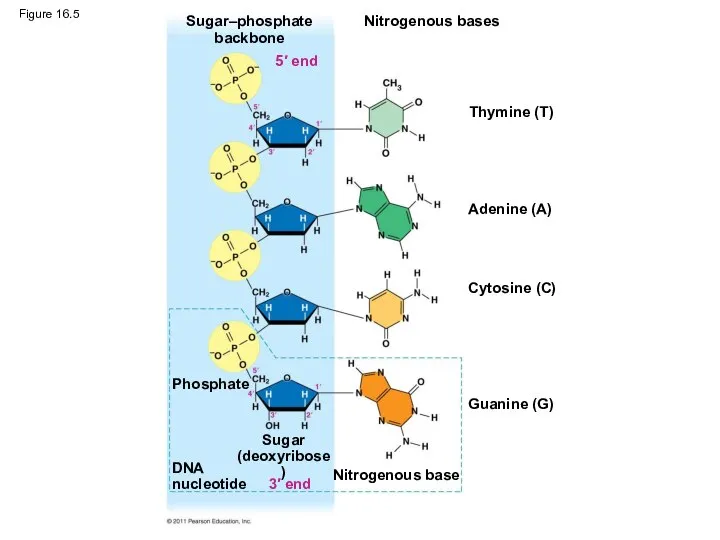
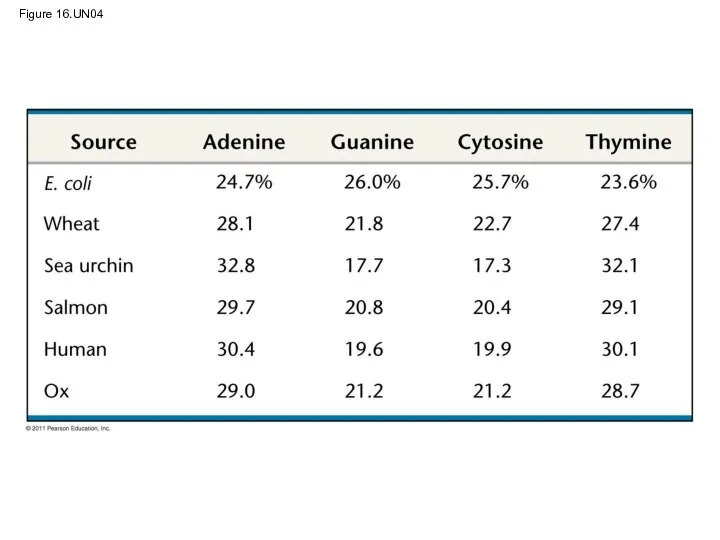
- 20. Two findings became known as Chargaff’s rules The base composition of DNA varies between species In
- 21. Figure 16.5 Sugar–phosphate backbone Nitrogenous bases Thymine (T) Adenine (A) Cytosine (C) Guanine (G) Nitrogenous base
- 22. Building a Structural Model of DNA: Scientific Inquiry After DNA was accepted as the genetic material,
- 23. Figure 16.6 (a) Rosalind Franklin
- 24. Figure 16.6a (a) Rosalind Franklin
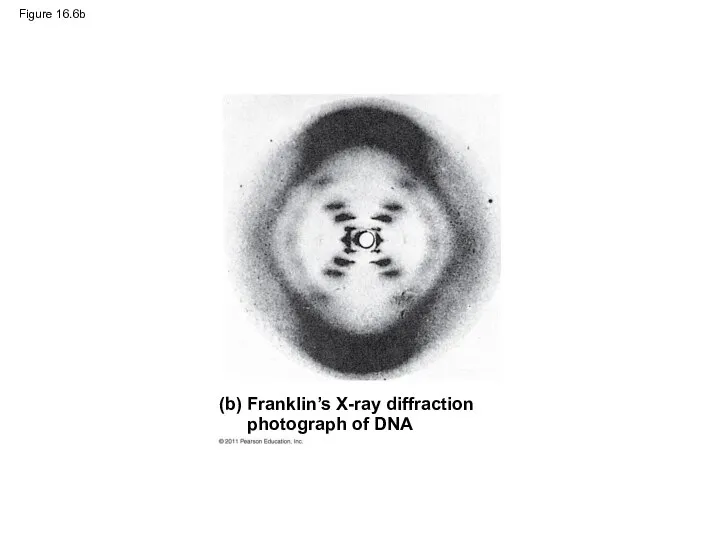
- 25. Figure 16.6b
- 26. Franklin’s X-ray crystallographic images of DNA enabled Watson to deduce that DNA was helical The X-ray
- 27. Animation: DNA Double Helix Right-click slide / select “Play”
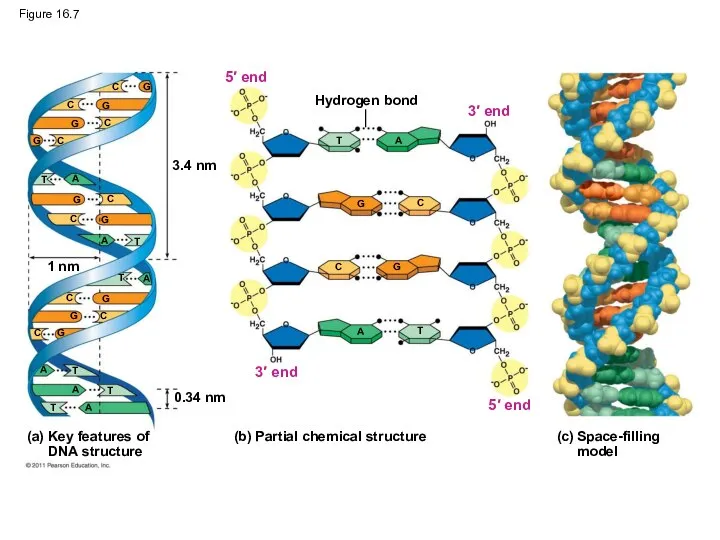
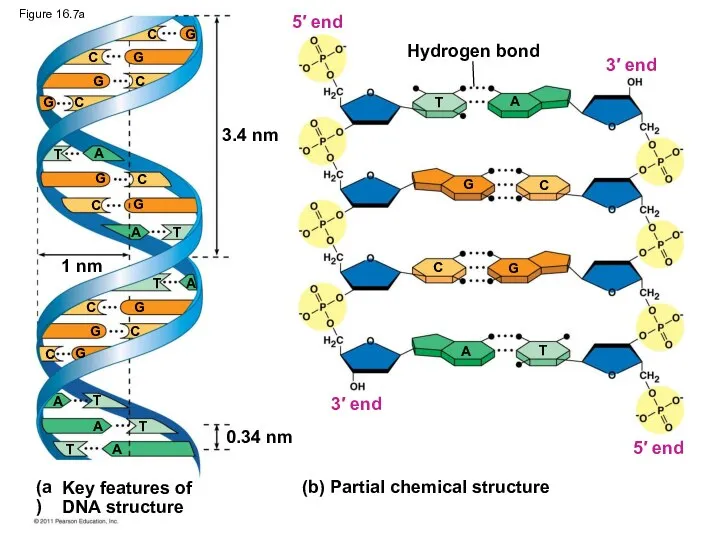
- 28. Figure 16.7 3.4 nm 1 nm 0.34 nm Hydrogen bond (b) Partial chemical structure 3′ end
- 29. 3.4 nm 1 nm 0.34 nm Hydrogen bond (b) Partial chemical structure 3′ end 5′ end
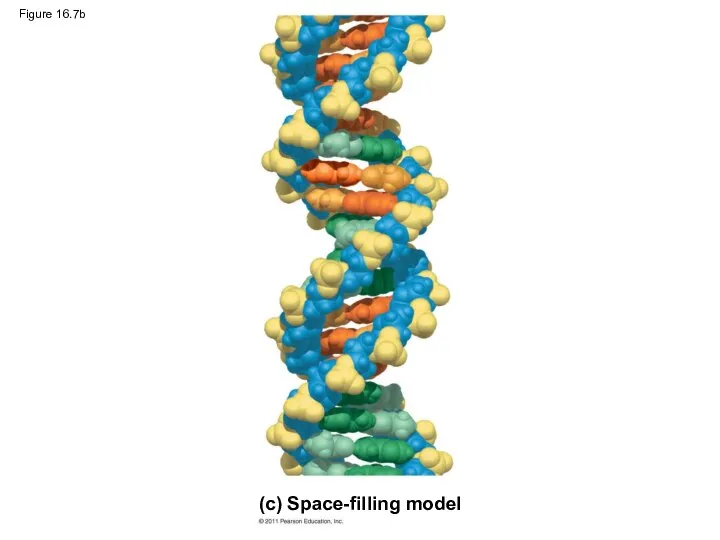
- 30. Figure 16.7b (c) Space-filling model
- 31. Watson and Crick built models of a double helix to conform to the X-rays and chemistry
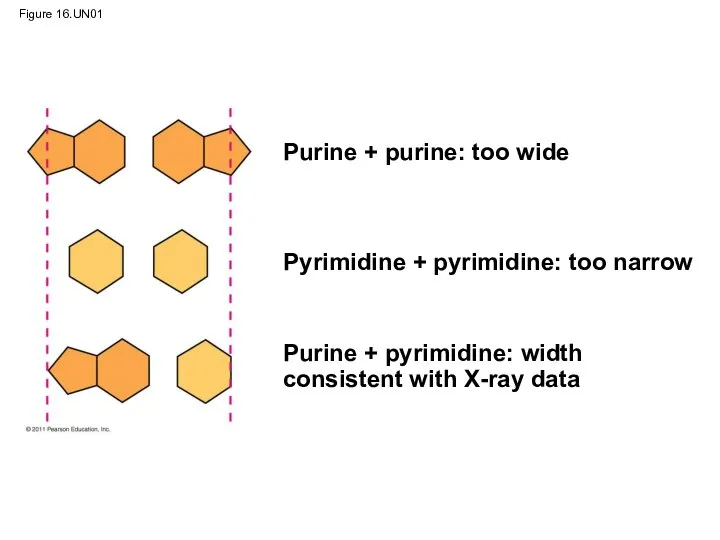
- 32. At first, Watson and Crick thought the bases paired like with like (A with A, and
- 33. Figure 16.UN01 Purine + purine: too wide Pyrimidine + pyrimidine: too narrow Purine + pyrimidine: width
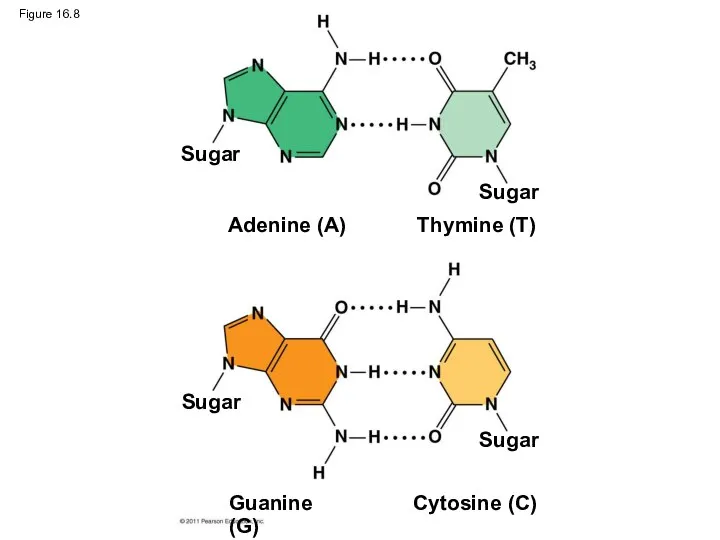
- 34. Watson and Crick reasoned that the pairing was more specific, dictated by the base structures They
- 35. Figure 16.8 Sugar Sugar Sugar Sugar Adenine (A) Thymine (T) Guanine (G) Cytosine (C)
- 36. Concept 16.2: Many proteins work together in DNA replication and repair The relationship between structure and
- 37. The Basic Principle: Base Pairing to a Template Strand Since the two strands of DNA are
- 38. Animation: DNA Replication Overview Right-click slide / select “Play”
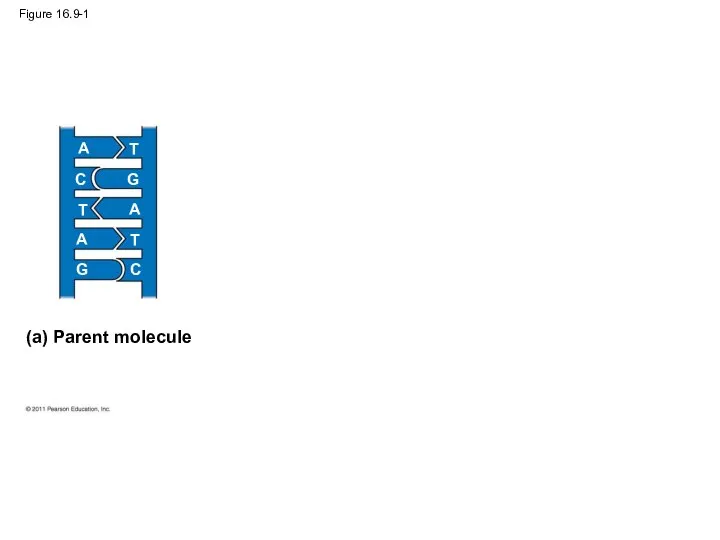
- 39. Figure 16.9-1 (a) Parent molecule A A A T T T C C G G
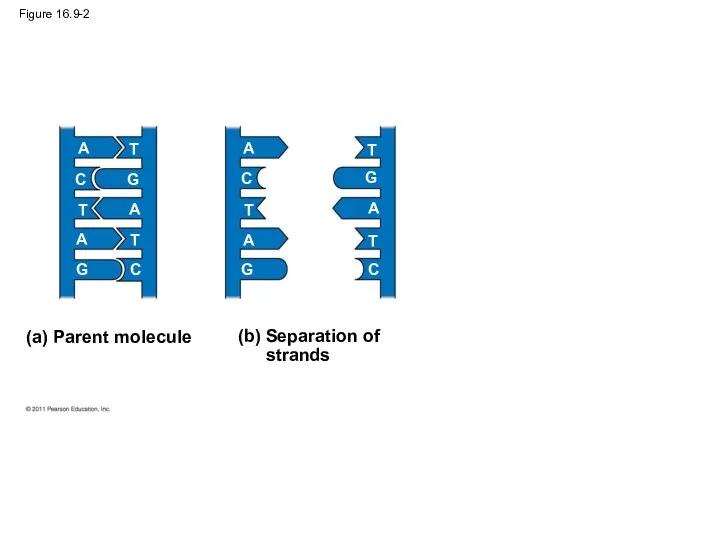
- 40. Figure 16.9-2 (a) Parent molecule A A A A A A T T T T T
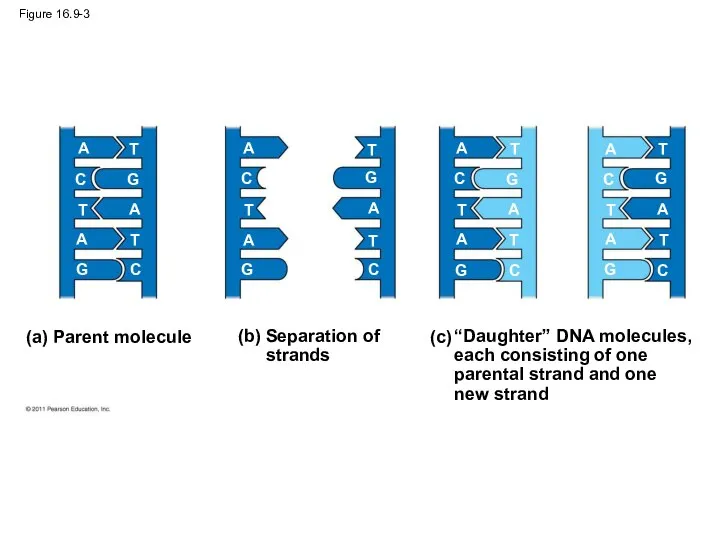
- 41. Figure 16.9-3 (a) Parent molecule A A A A A A A A A A A
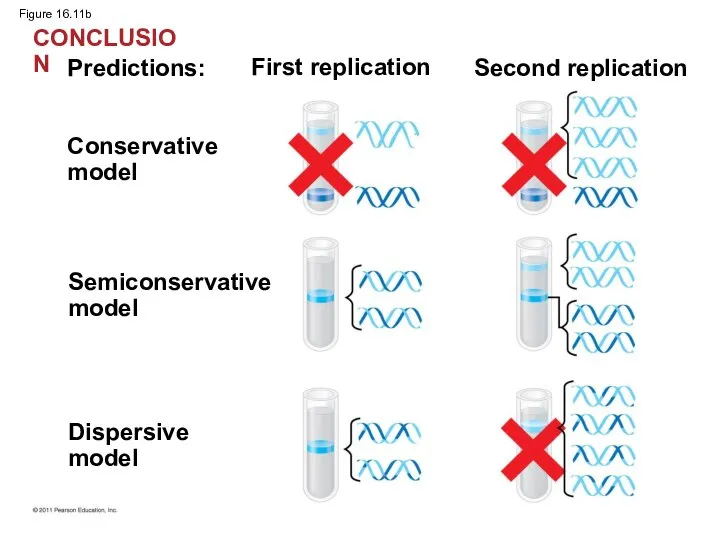
- 42. Watson and Crick’s semiconservative model of replication predicts that when a double helix replicates, each daughter
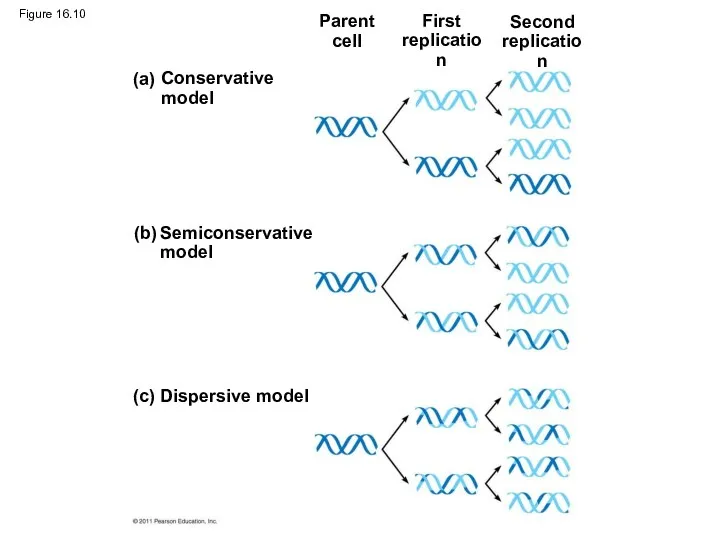
- 43. Figure 16.10 (c) Dispersive model Parent cell First replication Second replication
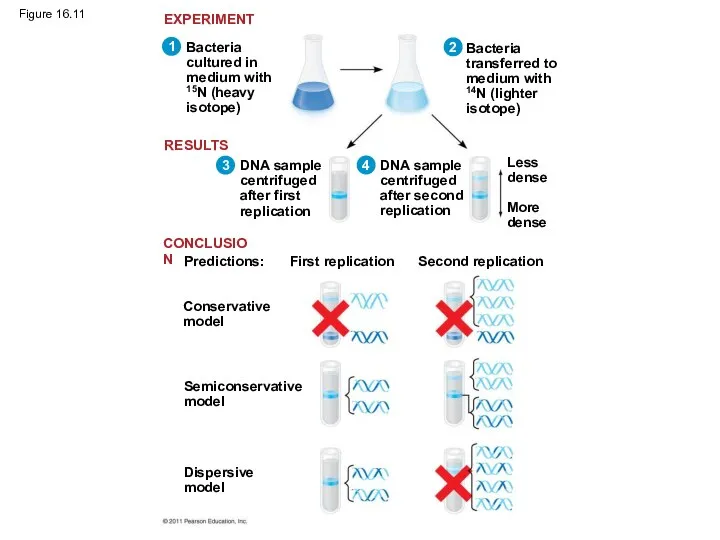
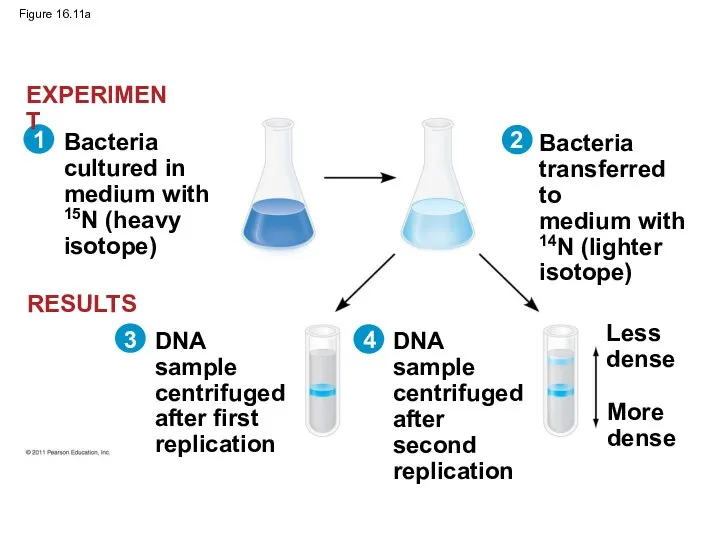
- 44. Experiments by Matthew Meselson and Franklin Stahl supported the semiconservative model They labeled the nucleotides of
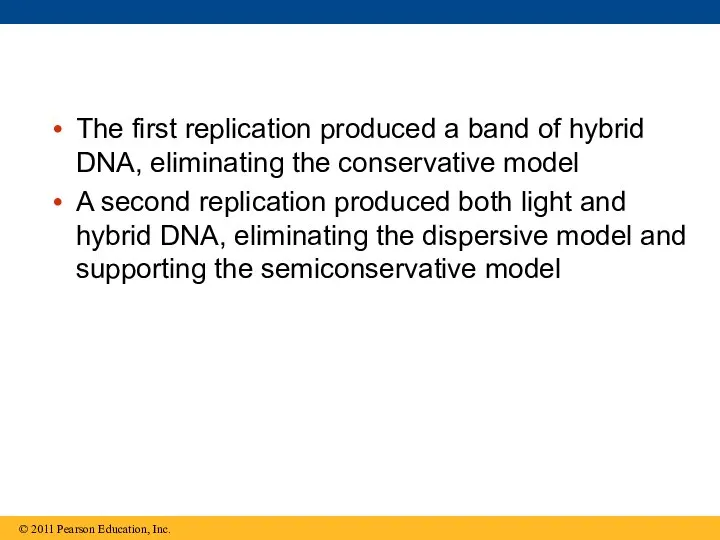
- 45. The first replication produced a band of hybrid DNA, eliminating the conservative model A second replication
- 46. Figure 16.11 Bacteria cultured in medium with 15N (heavy isotope) Bacteria transferred to medium with 14N
- 47. Figure 16.11a Bacteria cultured in medium with 15N (heavy isotope) Bacteria transferred to medium with 14N
- 48. Figure 16.11b Predictions: First replication Second replication Conservative model Semiconservative model Dispersive model CONCLUSION
- 49. DNA Replication: A Closer Look The copying of DNA is remarkable in its speed and accuracy
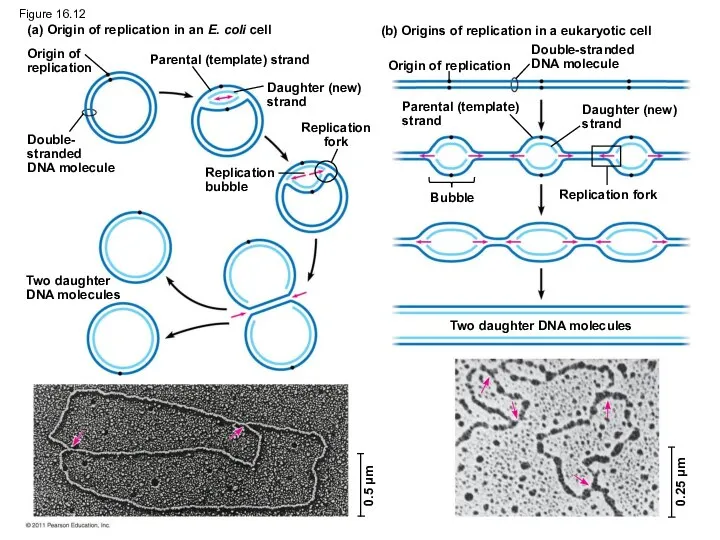
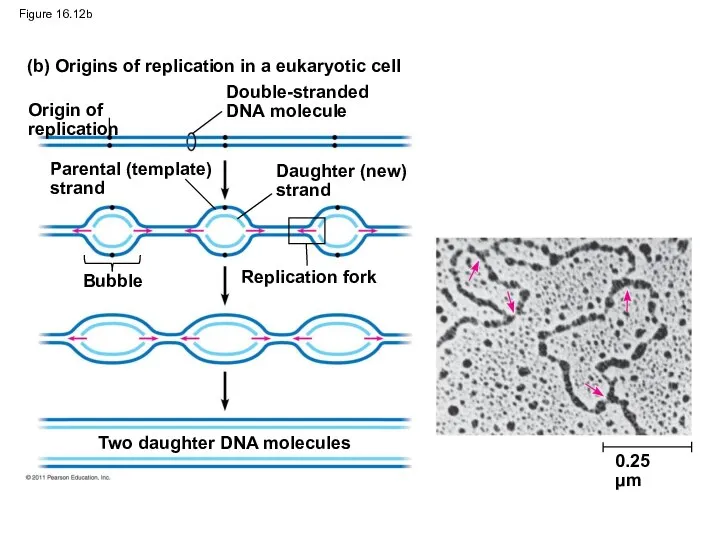
- 50. Getting Started Replication begins at particular sites called origins of replication, where the two DNA strands
- 51. Animation: Origins of Replication Right-click slide / select “Play”
- 52. Figure 16.12 (a) Origin of replication in an E. coli cell (b) Origins of replication in
- 53. Figure 16.12a (a) Origin of replication in an E. coli cell Origin of replication Parental (template)
- 54. Figure 16.12b (b) Origins of replication in a eukaryotic cell Origin of replication Double-stranded DNA molecule
- 55. Figure 16.12c 0.5 μm
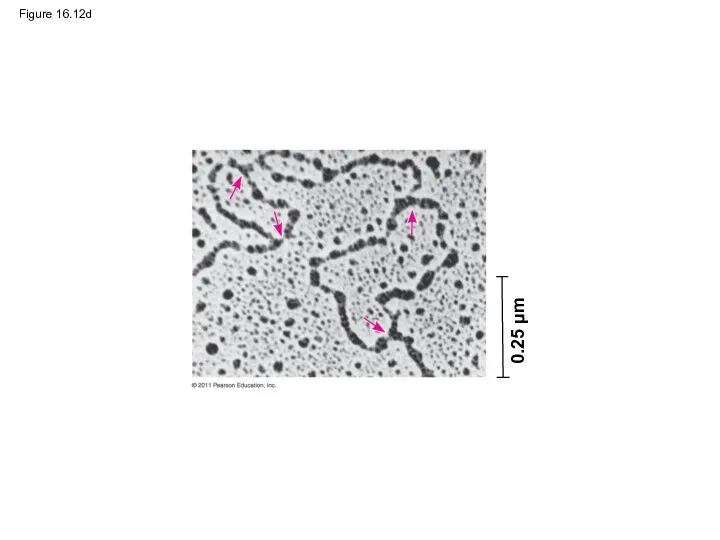
- 56. Figure 16.12d 0.25 μm
- 57. At the end of each replication bubble is a replication fork, a Y-shaped region where new
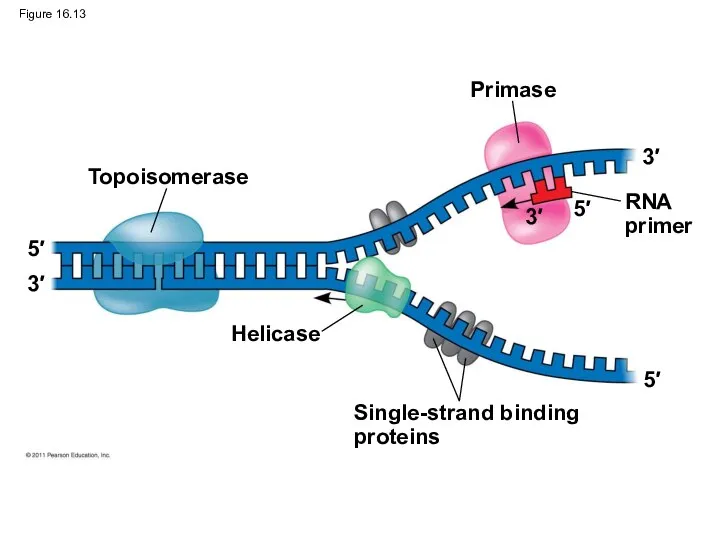
- 58. Figure 16.13 Topoisomerase Primase RNA primer Helicase Single-strand binding proteins 5′ 3′ 5′ 5′ 3′ 3′
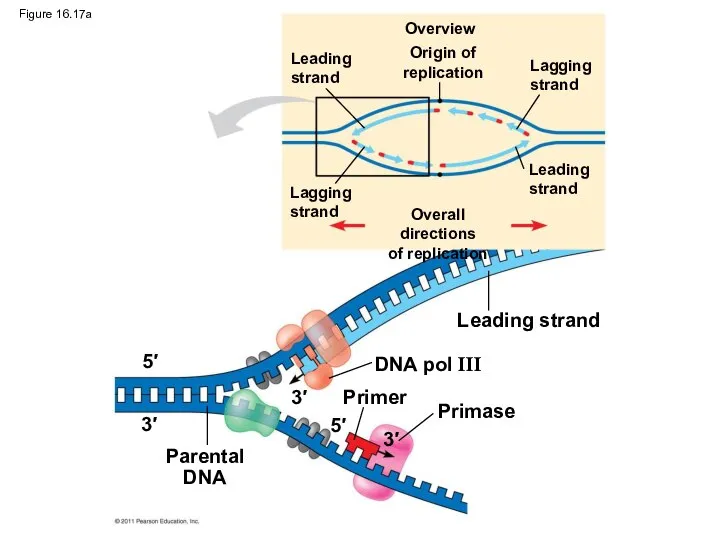
- 59. DNA polymerases cannot initiate synthesis of a polynucleotide; they can only add nucleotides to the 3′
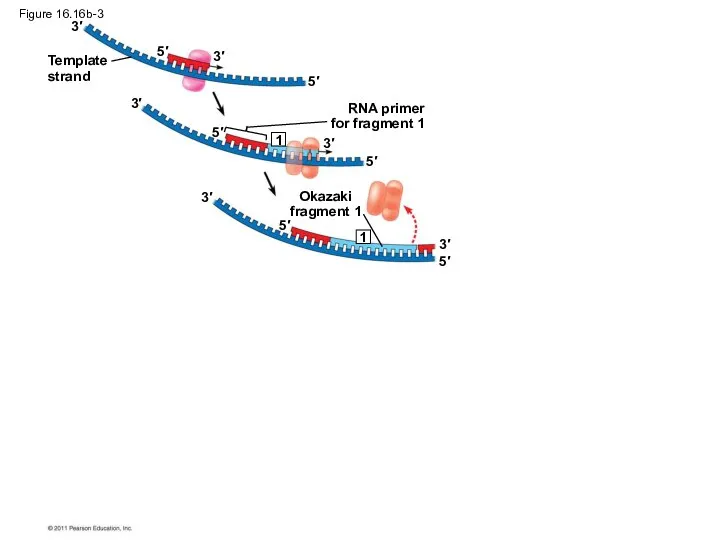
- 60. An enzyme called primase can start an RNA chain from scratch and adds RNA nucleotides one
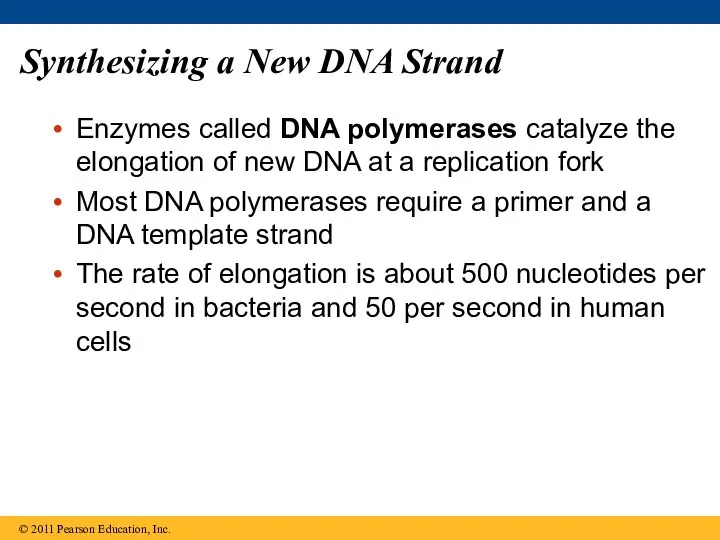
- 61. Synthesizing a New DNA Strand Enzymes called DNA polymerases catalyze the elongation of new DNA at
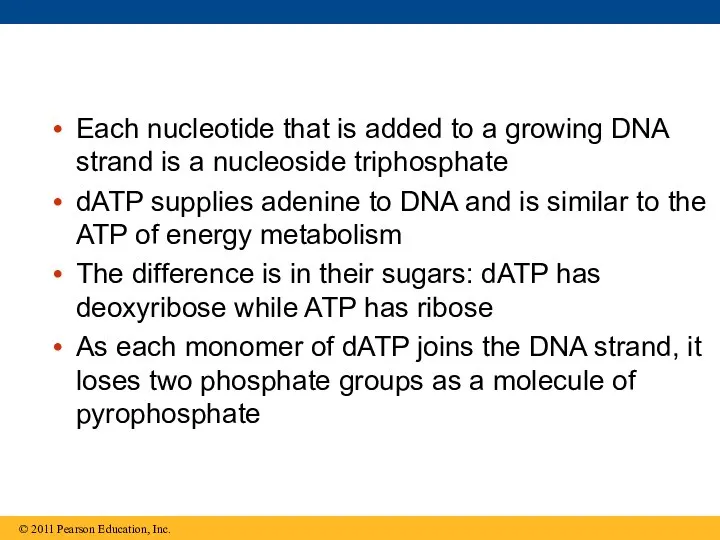
- 62. Each nucleotide that is added to a growing DNA strand is a nucleoside triphosphate dATP supplies
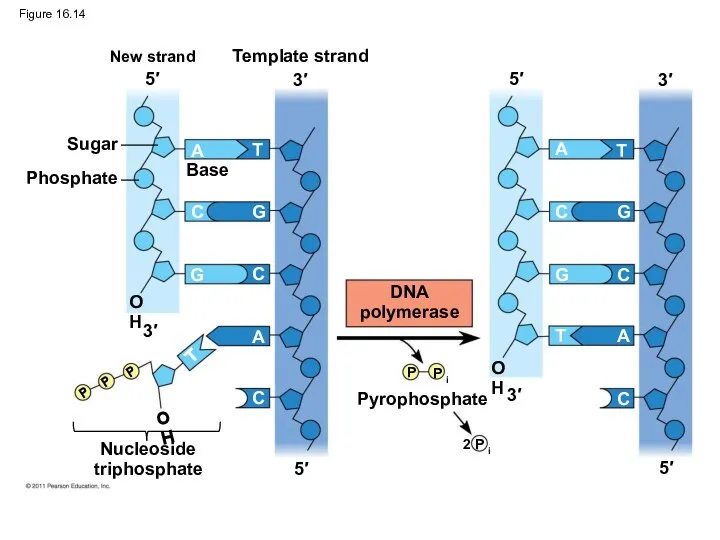
- 63. Figure 16.14 New strand Template strand Sugar Phosphate Base Nucleoside triphosphate DNA polymerase Pyrophosphate 5′ 5′
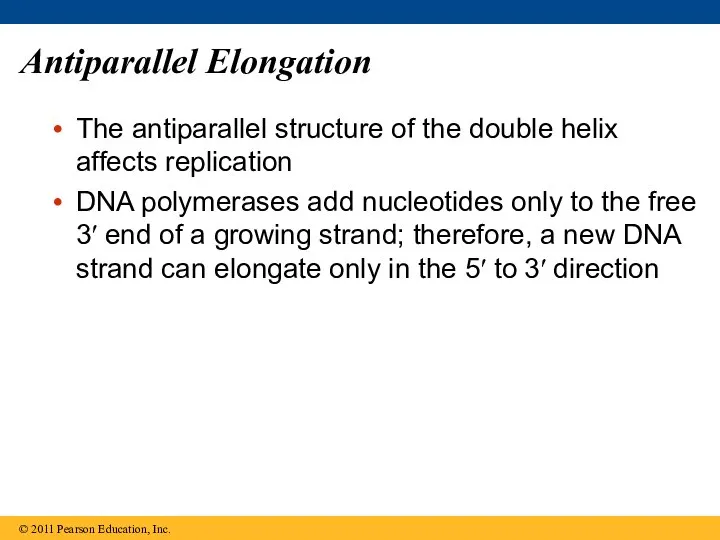
- 64. Antiparallel Elongation The antiparallel structure of the double helix affects replication DNA polymerases add nucleotides only
- 65. Along one template strand of DNA, the DNA polymerase synthesizes a leading strand continuously, moving toward
- 66. Animation: Leading Strand Right-click slide / select “Play”
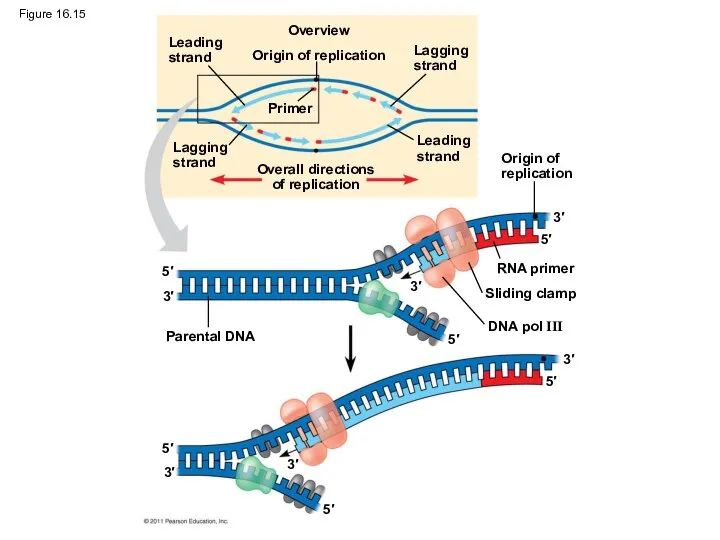
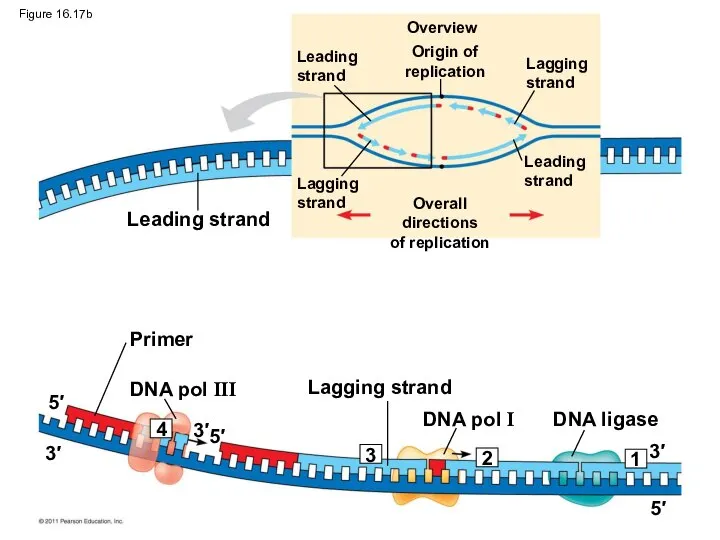
- 67. Figure 16.15 Leading strand Lagging strand Overview Origin of replication Lagging strand Leading strand Primer Overall
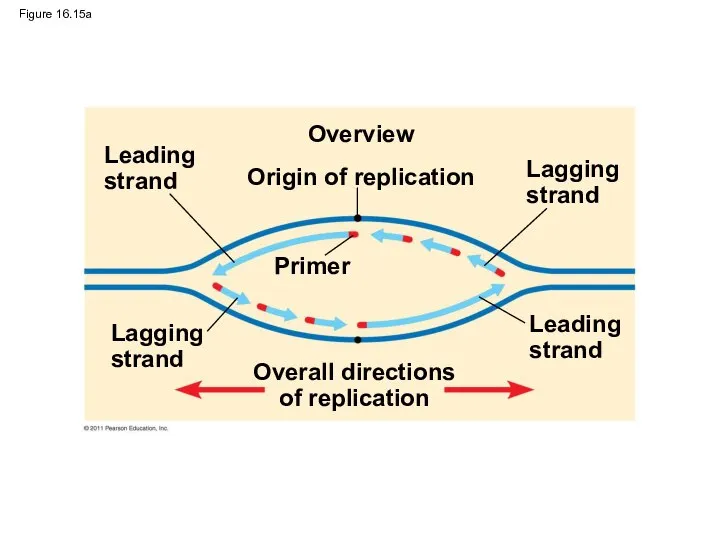
- 68. Figure 16.15a Leading strand Lagging strand Overview Origin of replication Lagging strand Leading strand Primer Overall
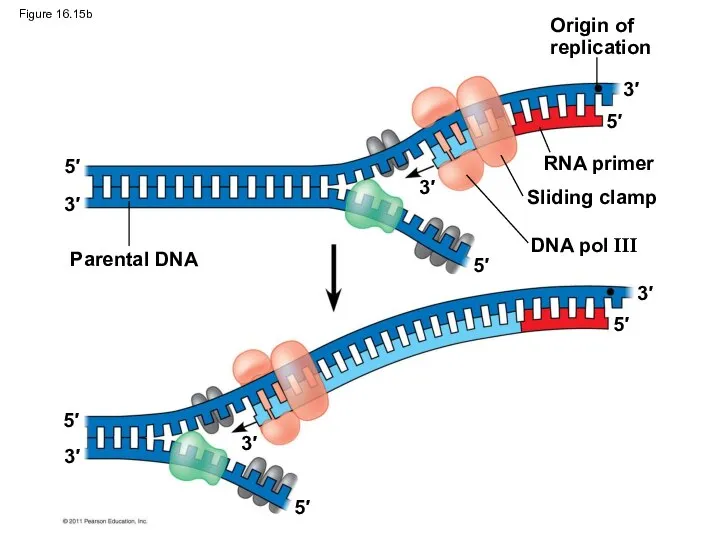
- 69. Origin of replication RNA primer Sliding clamp DNA pol III Parental DNA 3′ 5′ 5′ 3′
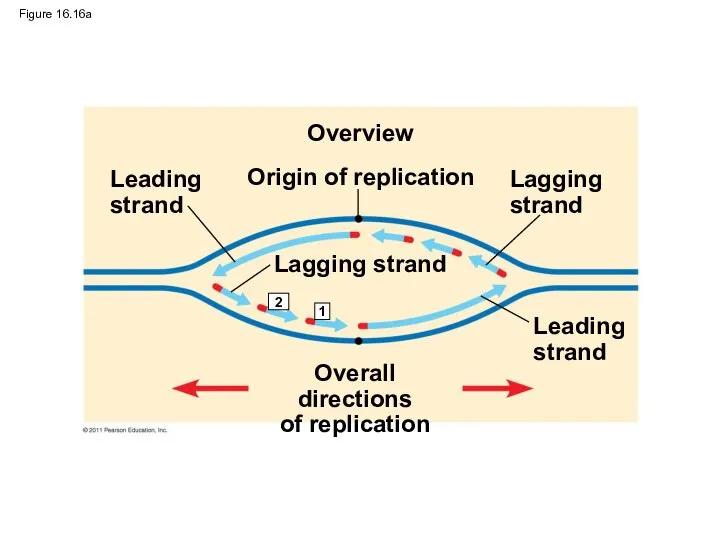
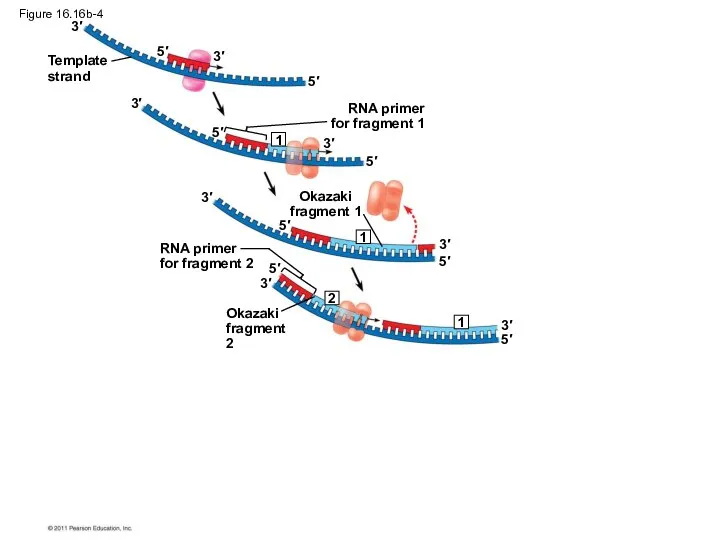
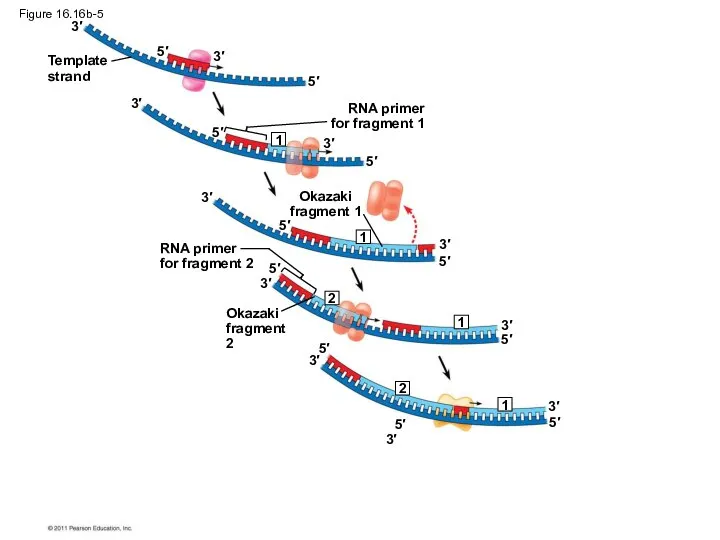
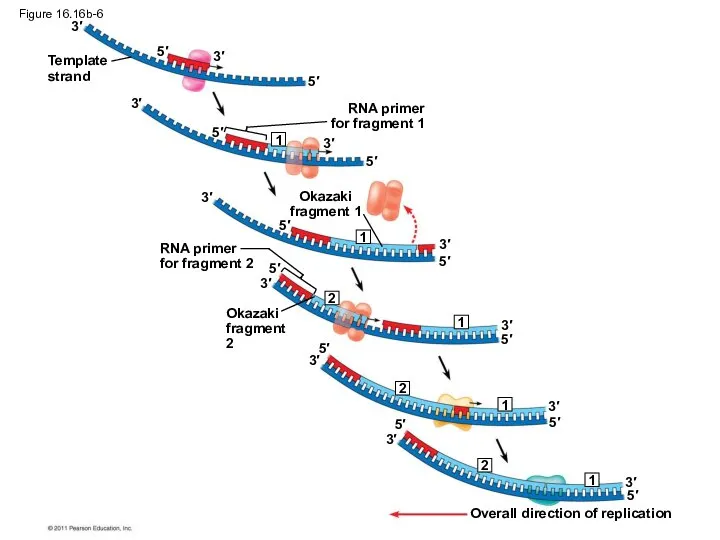
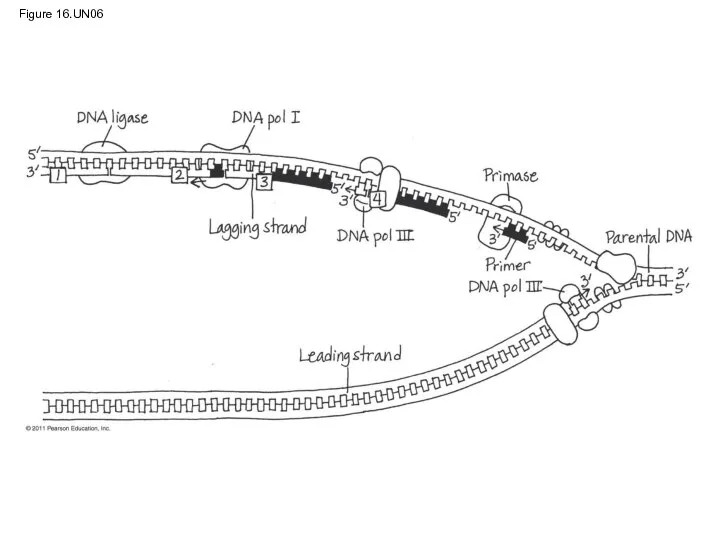
- 70. To elongate the other new strand, called the lagging strand, DNA polymerase must work in the
- 71. Animation: Lagging Strand Right-click slide / select “Play”
- 72. Origin of replication Overview Leading strand Leading strand Lagging strand Lagging strand Overall directions of replication
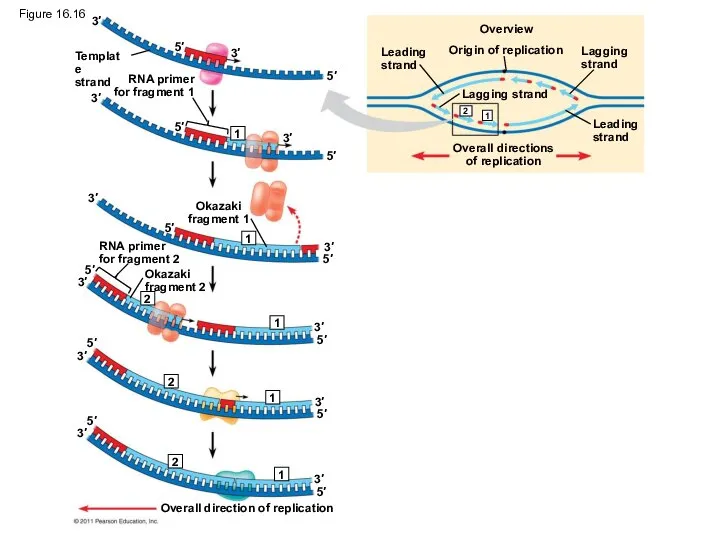
- 73. Figure 16.16a Origin of replication Overview Leading strand Leading strand Lagging strand Lagging strand Overall directions
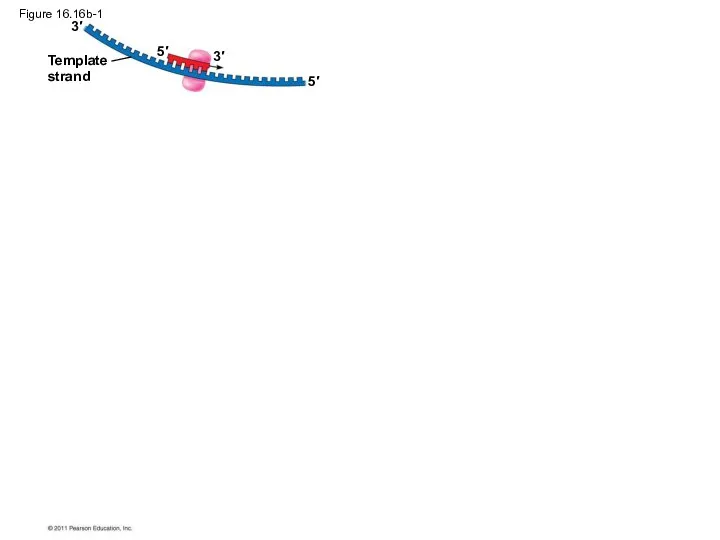
- 74. Figure 16.16b-1 Template strand 3′ 3′ 5′ 5′
- 75. Figure 16.16b-2 Template strand RNA primer for fragment 1 3′ 3′ 3′ 3′ 5′ 5′ 5′
- 76. Figure 16.16b-3 Template strand RNA primer for fragment 1 Okazaki fragment 1 3′ 3′ 3′ 3′
- 77. Figure 16.16b-4 Template strand RNA primer for fragment 1 Okazaki fragment 1 RNA primer for fragment
- 78. Figure 16.16b-5 Template strand RNA primer for fragment 1 Okazaki fragment 1 RNA primer for fragment
- 79. Figure 16.16b-6 Template strand RNA primer for fragment 1 Okazaki fragment 1 RNA primer for fragment
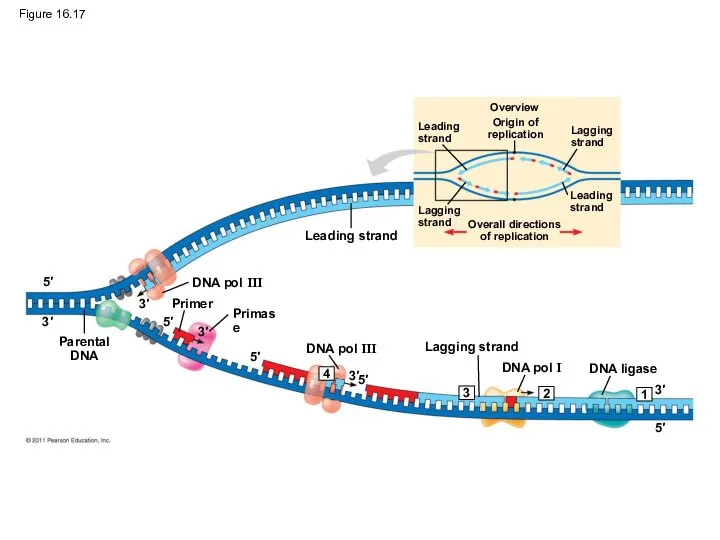
- 80. Figure 16.17 Overview Leading strand Origin of replication Lagging strand Leading strand Lagging strand Overall directions
- 81. Figure 16.17a Overview Leading strand Origin of replication Lagging strand Leading strand Lagging strand Overall directions
- 82. Overview Leading strand Origin of replication Lagging strand Leading strand Lagging strand Overall directions of replication
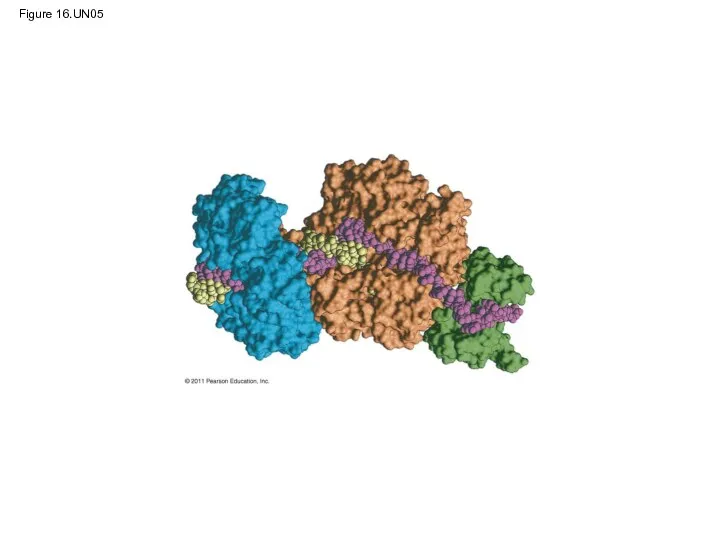
- 83. The DNA Replication Complex The proteins that participate in DNA replication form a large complex, a
- 84. Animation: DNA Replication Review Right-click slide / select “Play”
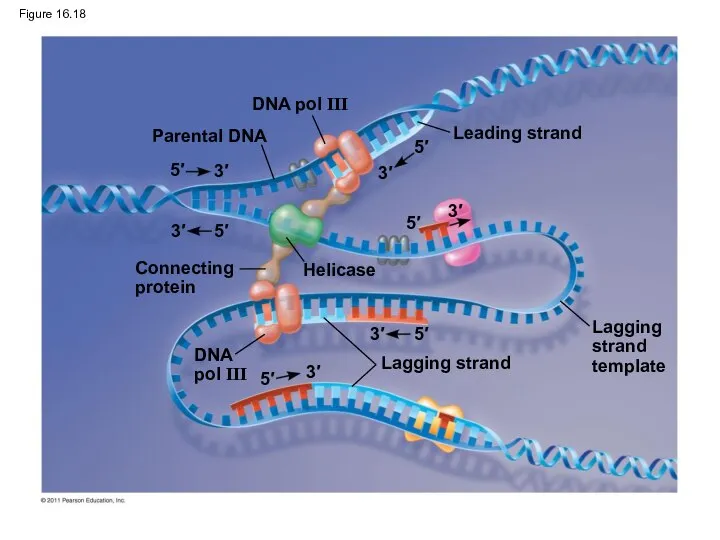
- 85. Figure 16.18 Parental DNA DNA pol III Leading strand Connecting protein Helicase Lagging strand DNA pol
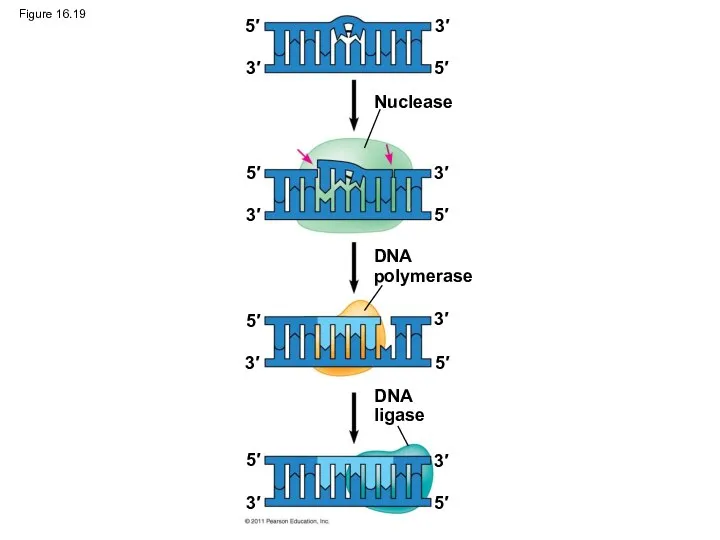
- 86. Proofreading and Repairing DNA DNA polymerases proofread newly made DNA, replacing any incorrect nucleotides In mismatch
- 87. Figure 16.19 Nuclease DNA polymerase DNA ligase 5′ 5′ 5′ 5′ 5′ 5′ 5′ 5′ 3′
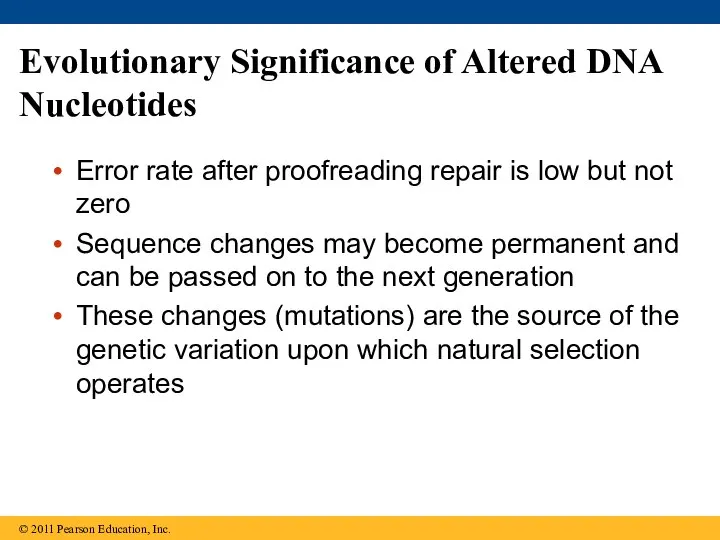
- 88. Evolutionary Significance of Altered DNA Nucleotides Error rate after proofreading repair is low but not zero
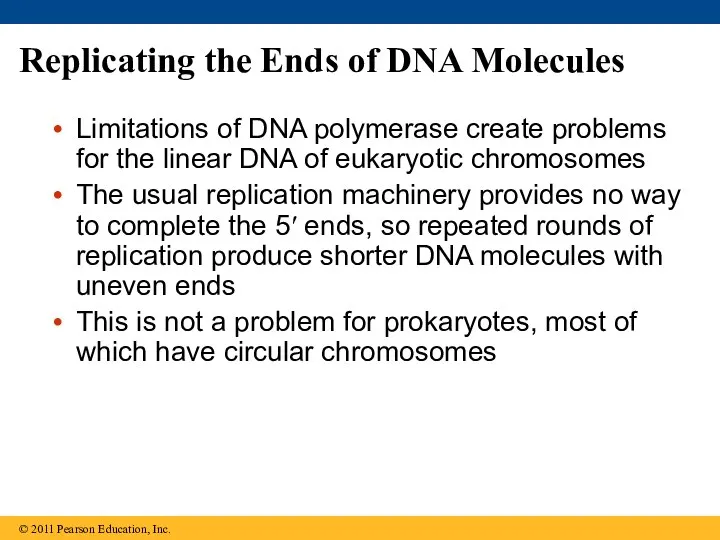
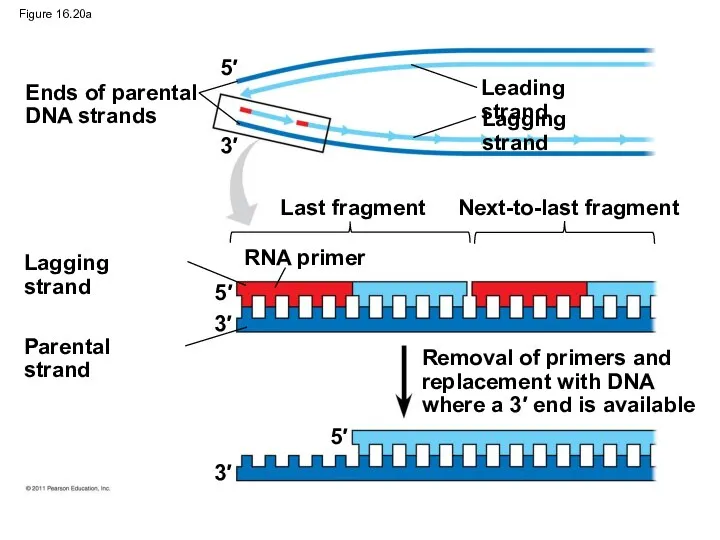
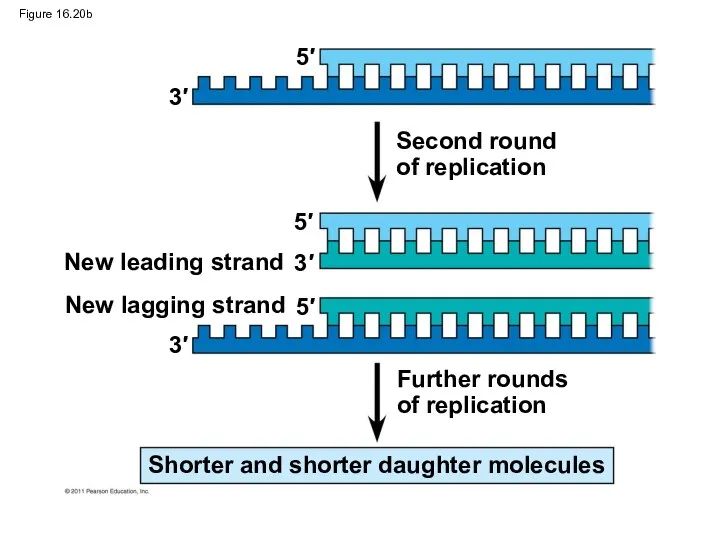
- 89. Replicating the Ends of DNA Molecules Limitations of DNA polymerase create problems for the linear DNA
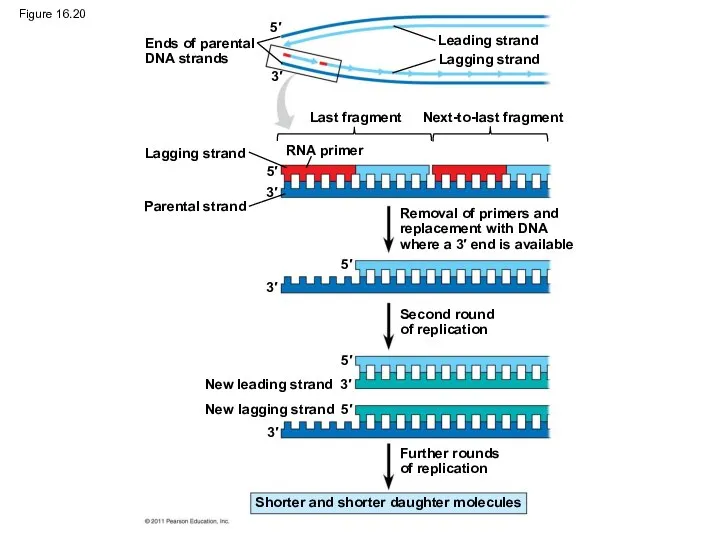
- 90. Figure 16.20 Ends of parental DNA strands Leading strand Lagging strand Last fragment Next-to-last fragment Lagging
- 91. Figure 16.20a Ends of parental DNA strands Leading strand Lagging strand Last fragment Next-to-last fragment Lagging
- 92. Figure 16.20b Second round of replication Further rounds of replication New leading strand New lagging strand
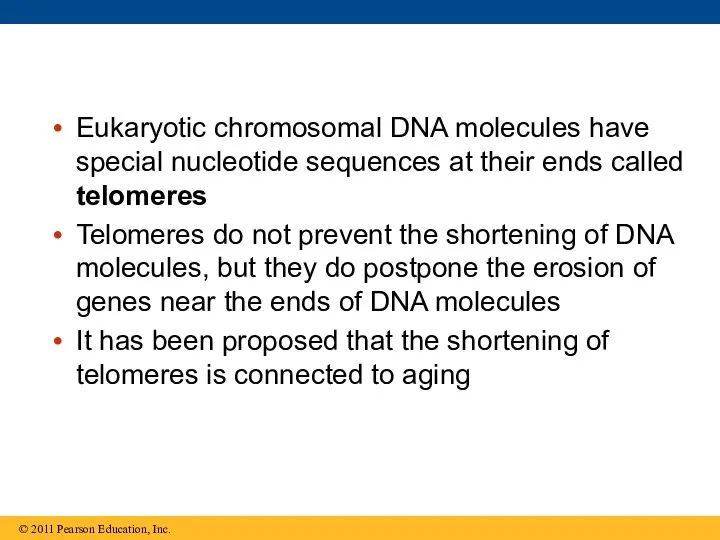
- 93. Eukaryotic chromosomal DNA molecules have special nucleotide sequences at their ends called telomeres Telomeres do not
- 94. Figure 16.21 1 μm
- 95. If chromosomes of germ cells became shorter in every cell cycle, essential genes would eventually be
- 96. The shortening of telomeres might protect cells from cancerous growth by limiting the number of cell
- 97. Concept 16.3 A chromosome consists of a DNA molecule packed together with proteins The bacterial chromosome
- 98. Chromatin, a complex of DNA and protein, is found in the nucleus of eukaryotic cells Chromosomes
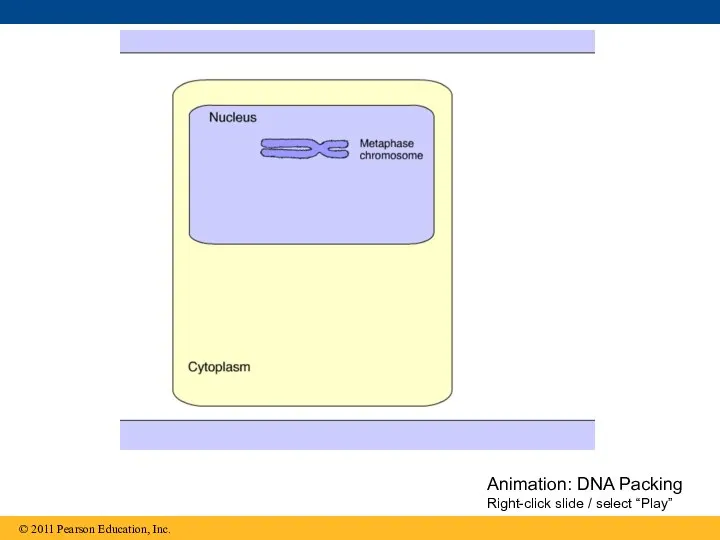
- 99. Animation: DNA Packing Right-click slide / select “Play”
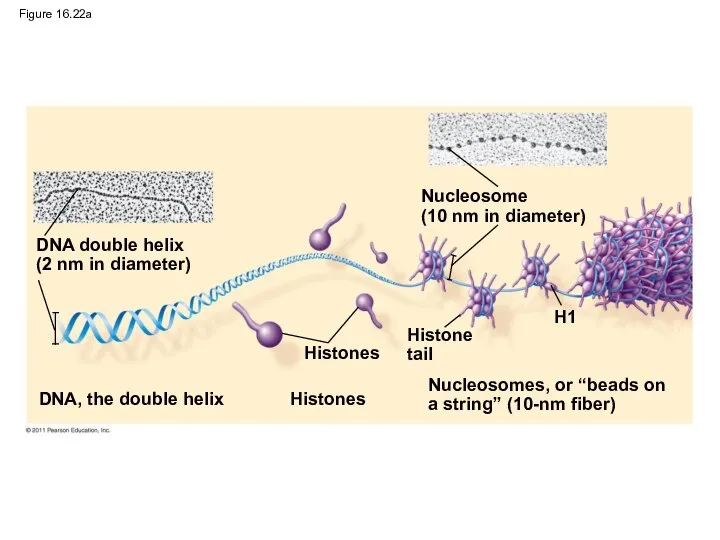
- 100. Figure 16.22a DNA double helix (2 nm in diameter) DNA, the double helix Nucleosome (10 nm
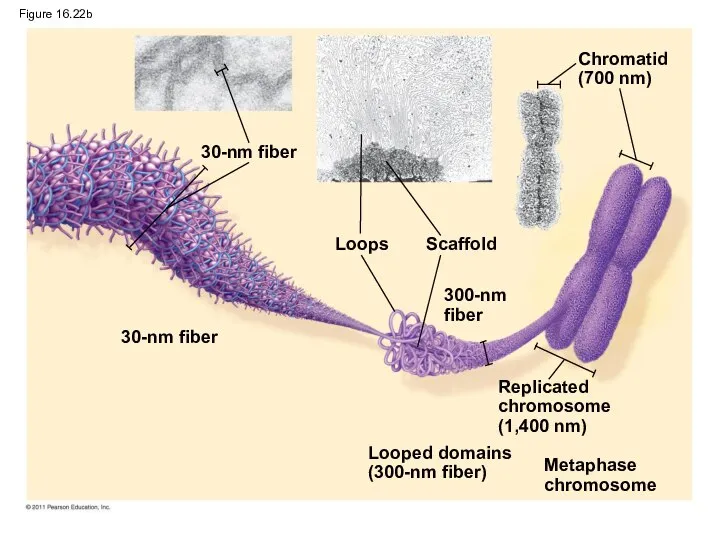
- 101. Figure 16.22b 30-nm fiber 30-nm fiber Loops Scaffold 300-nm fiber Chromatid (700 nm) Replicated chromosome (1,400
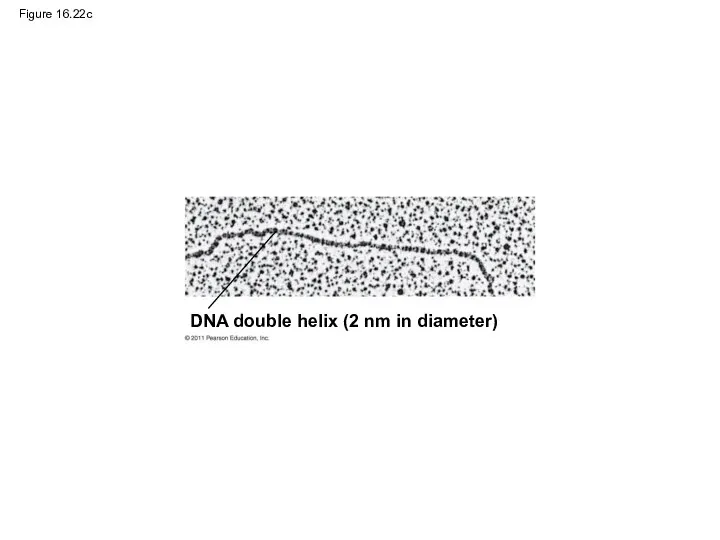
- 102. Figure 16.22c DNA double helix (2 nm in diameter)
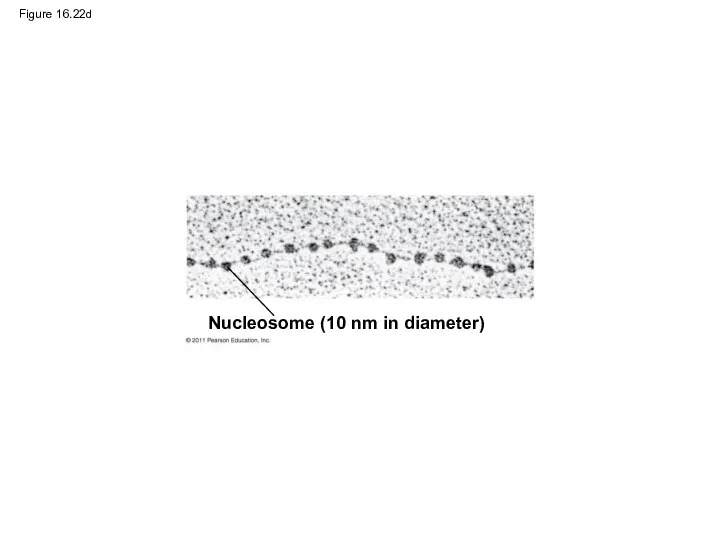
- 103. Figure 16.22d Nucleosome (10 nm in diameter)
- 104. Figure 16.22e 30-nm fiber
- 105. Figure 16.22f Loops Scaffold
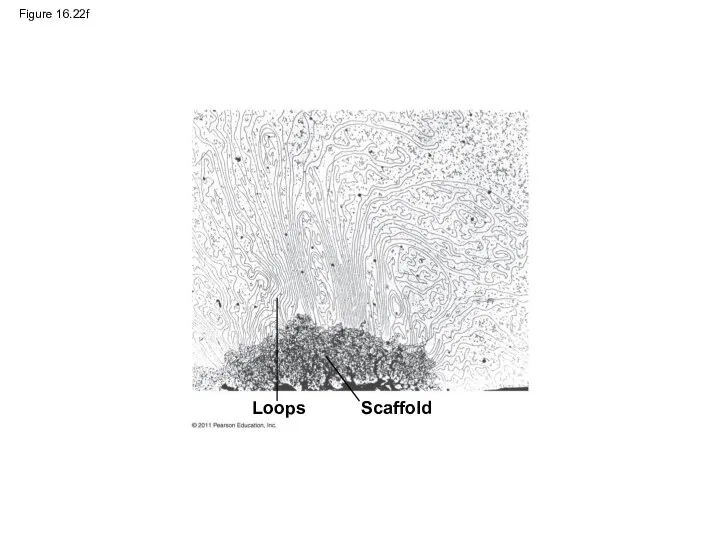
- 106. Figure 16.22g Chromatid (700 nm)
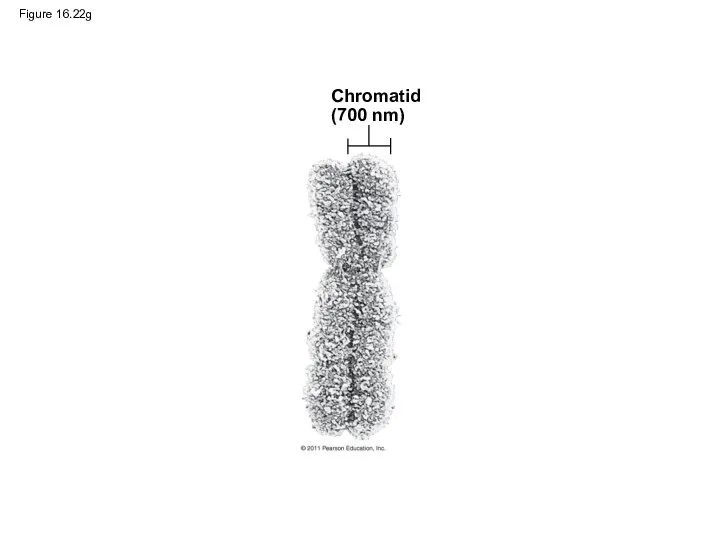
- 107. Chromatin undergoes changes in packing during the cell cycle At interphase, some chromatin is organized into
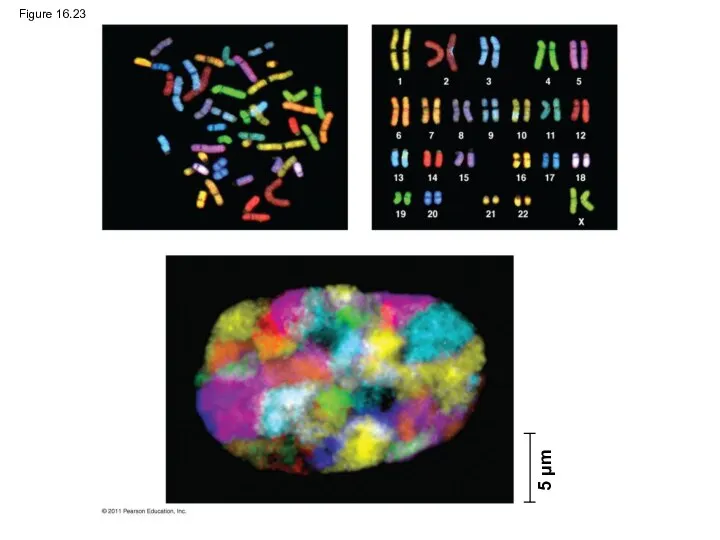
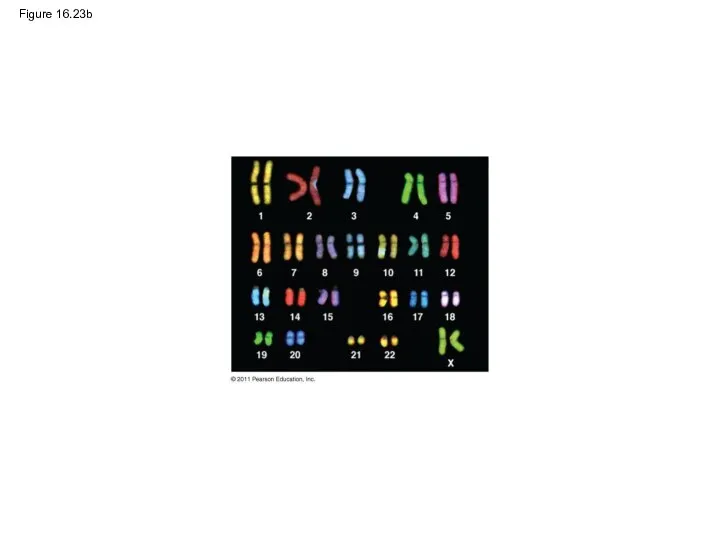
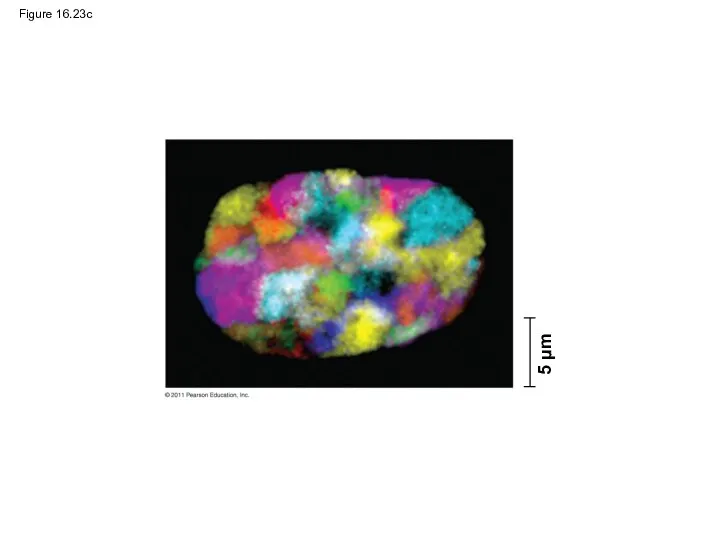
- 108. Figure 16.23 5 μm
- 109. Figure 16.23a
- 110. Figure 16.23b
- 111. Figure 16.23c 5 μm
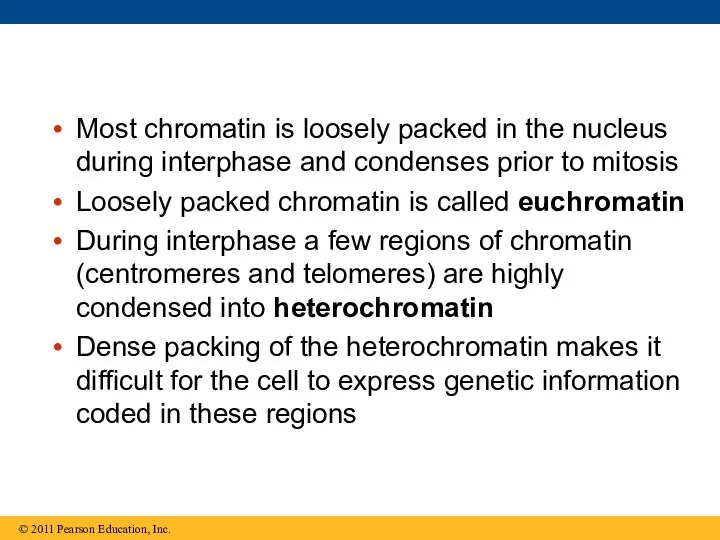
- 112. Most chromatin is loosely packed in the nucleus during interphase and condenses prior to mitosis Loosely
- 113. Histones can undergo chemical modifications that result in changes in chromatin organization
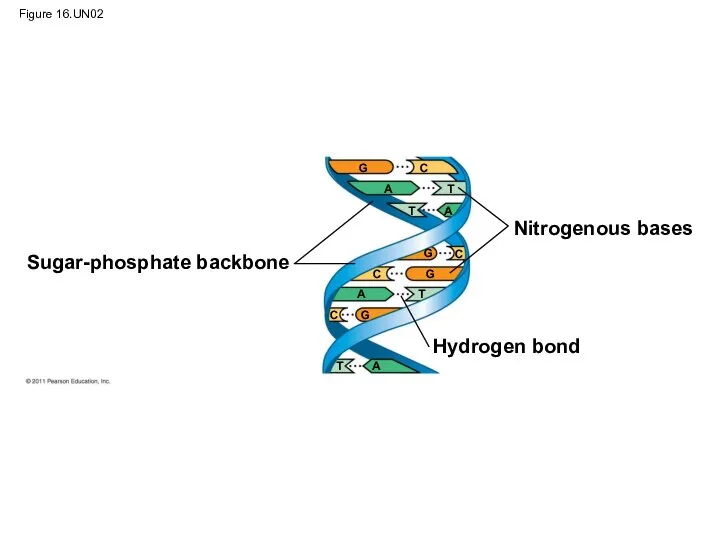
- 114. Figure 16.UN02 Sugar-phosphate backbone Nitrogenous bases Hydrogen bond G G G G C C C C
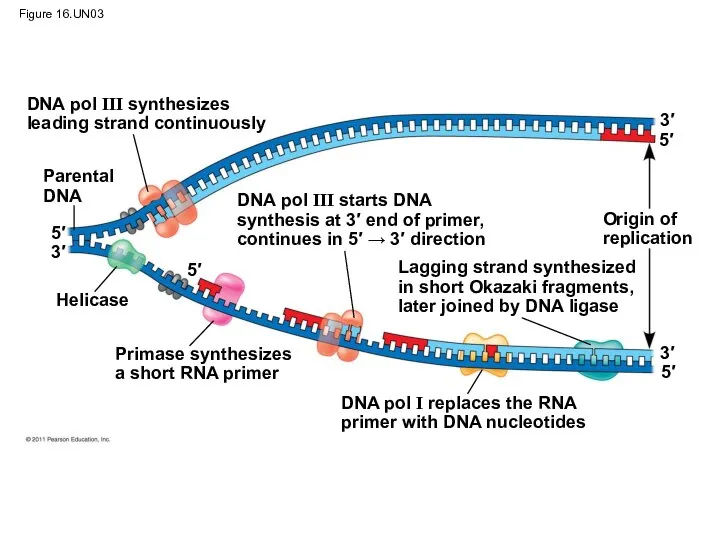
- 115. Figure 16.UN03 DNA pol III synthesizes leading strand continuously Parental DNA DNA pol III starts DNA
- 116. Figure 16.UN04
- 117. Figure 16.UN05
- 118. Figure 16.UN06
- 120. Скачать презентацию





































 Вода. Классы неорганических соединений. 8 класс
Вода. Классы неорганических соединений. 8 класс Вакуумное фильтрование
Вакуумное фильтрование Битумы природного происхождения
Битумы природного происхождения Растворение. Растворы
Растворение. Растворы Вещества
Вещества Пропан, C3H8
Пропан, C3H8 Презентация по Химии "Соединения" - скачать смотреть бесплатно
Презентация по Химии "Соединения" - скачать смотреть бесплатно Минералы и Близнецы
Минералы и Близнецы Озоновый слой. Механизмы образования и разрушения
Озоновый слой. Механизмы образования и разрушения Химически опасные объекты (ХОО)
Химически опасные объекты (ХОО) Химические реакции (11 класс)
Химические реакции (11 класс) Диазо- и азосоединения
Диазо- и азосоединения Структура кристаллических полимеров
Структура кристаллических полимеров Положение металлов в Периодической системе Д.И. Менделеева. Особенности строения атомов, свойства
Положение металлов в Периодической системе Д.И. Менделеева. Особенности строения атомов, свойства Железо. Физические и химические свойства
Железо. Физические и химические свойства Презентация по Химии "ЖИРНЫЕ КИСЛОТЫ СОСТАВНЫЕ ЧАСТИ ЛИПИДОВ" - скачать смотреть бесплатно
Презентация по Химии "ЖИРНЫЕ КИСЛОТЫ СОСТАВНЫЕ ЧАСТИ ЛИПИДОВ" - скачать смотреть бесплатно Работу выполнила: Ученица 9 класса ГОУ Лицея № 1524 Г.Москвы Себко Екатерина Научный руководитель: Учитель химии ГОУ Лицея № 1524 К
Работу выполнила: Ученица 9 класса ГОУ Лицея № 1524 Г.Москвы Себко Екатерина Научный руководитель: Учитель химии ГОУ Лицея № 1524 К Методы светорассеяния для исследования растворов (био) полимеров и наночастиц
Методы светорассеяния для исследования растворов (био) полимеров и наночастиц Презентация по Химии "Семь доисторических металлов" - скачать смотреть
Презентация по Химии "Семь доисторических металлов" - скачать смотреть  Окислительно-восстановительные реакции
Окислительно-восстановительные реакции Shapes of molecules
Shapes of molecules Над проектом работали ученики 6 класса: Над проектом работали ученики 6 класса: Крючков Слава Орлов Слава Старова Катя Пугачев Се
Над проектом работали ученики 6 класса: Над проектом работали ученики 6 класса: Крючков Слава Орлов Слава Старова Катя Пугачев Се Статья двумя способами. Приготовление катализатора
Статья двумя способами. Приготовление катализатора Кислотно-основные взаимодействия. Принцип ЖМКО Пирсона
Кислотно-основные взаимодействия. Принцип ЖМКО Пирсона Оксиды азота
Оксиды азота Тест по теме «Альдегиды и кетоны»
Тест по теме «Альдегиды и кетоны» Тұздар. Құрамы және химиялық қасиеттері
Тұздар. Құрамы және химиялық қасиеттері Химическая очистка воды
Химическая очистка воды