Содержание
- 2. TERMINOLOGY A malignant proliferation of monoclonal hematopoetic cells with accumulation of abnormal immature cells which replace
- 3. Leukemias A very heterogeneic group of disorders, can be classified on a basis of clinical course
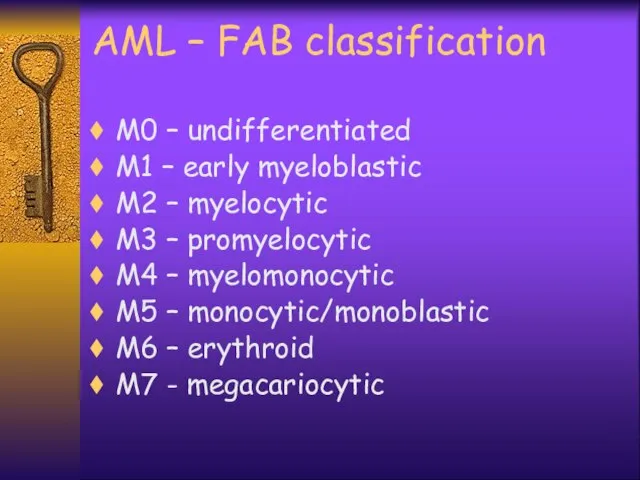
- 4. AML – FAB classification M0 – undifferentiated M1 – early myeloblastic M2 – myelocytic M3 –
- 5. FAB classification This classification is based mostly on morphology and immunophenotyping of the blasts Has clinical
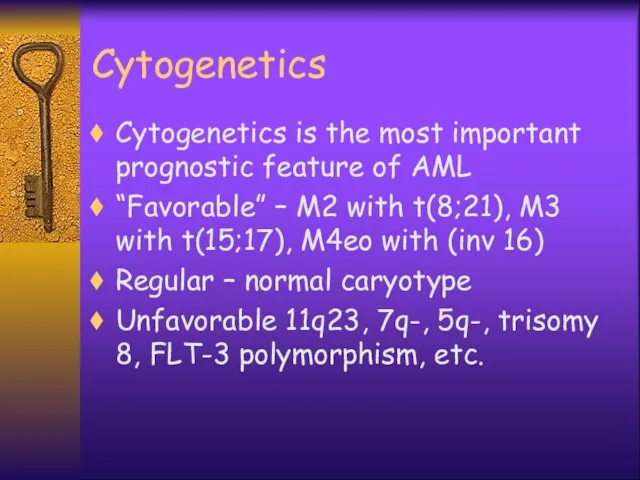
- 6. Cytogenetics Cytogenetics is the most important prognostic feature of AML “Favorable” – M2 with t(8;21), M3
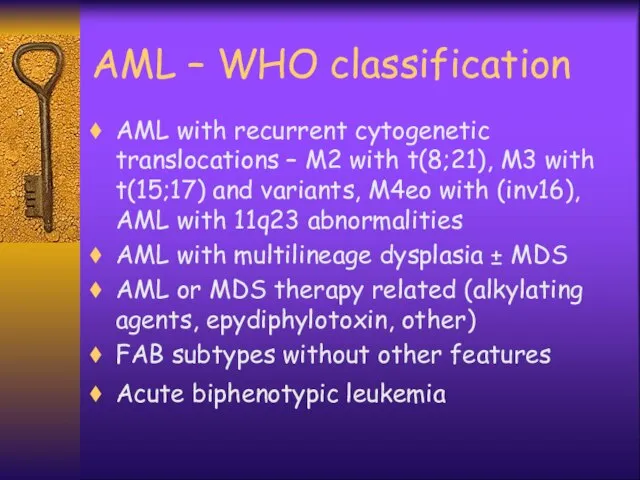
- 7. AML – WHO classification AML with recurrent cytogenetic translocations – M2 with t(8;21), M3 with t(15;17)
- 8. ALL The FAB classification is not in use Is classified by the phenotype of the blasts
- 9. ETIOLOGY Environment: irradiation, chemotherapeutic agents, organic solvents – benzene etc. Genetic diseases: neurofibromatosis, Wiscott-Aldrich synd., defective
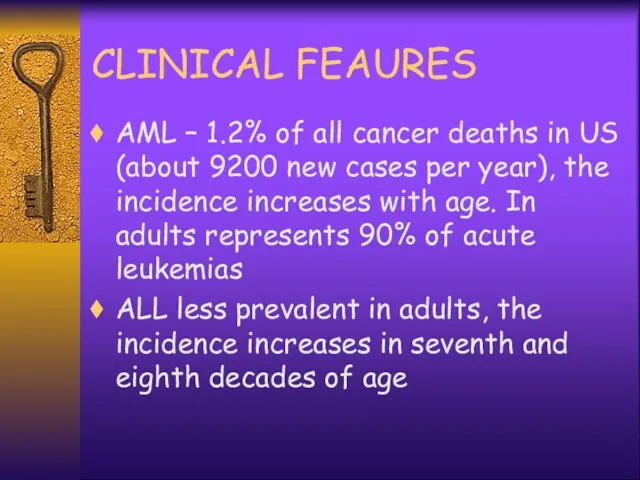
- 10. CLINICAL FEAURES AML – 1.2% of all cancer deaths in US (about 9200 new cases per
- 11. CLINICAL FEATURES The presenting signs are not specific: Anemia – pallor, weakness, dispnoea Neutropenia – fever,
- 12. LABORATORY Leukocytosis with blasts Metabolic and electrolyte derangement hyperuricemia, hyperkalemia, hyperphosphatemia – tumor lysis syndrome Coagulopathy
- 13. DIAGNOSIS Blasts in blood or bone marrow smear, Auer rods pathognomonic to AML Immunohistochemistry – peroxidase
- 14. Immunophenotyping CD – Cluster Designation, molecules on the surface of the cell, characteristic to each cell
- 15. AML - TREATMENT AML – induction with ARA-C and daunorubicin (7:3); consolidations with HIDAC and others,
- 16. ALL - TREATMENT Protocols based on treatment of childhood ALL, prolonged and intensive therapy with CNS
- 17. CML A clonal expansion of hematopoetic progenitors, characterized clinically by myeloid hyperplasia, leukocytosis with basophilia and
- 18. C M L A phasic disease – chronic phase, accelerated phase, blast crisis Incidence – 1-2:100000
- 19. CML - cytogenetics The first malignancy in which the link between a chromosomal abnormality and leukemogenesis
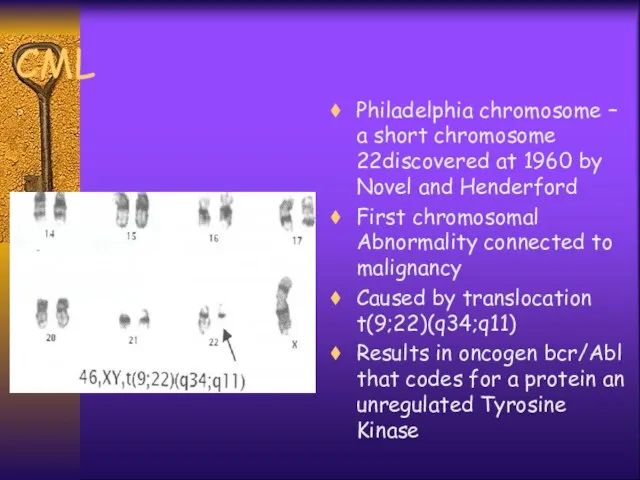
- 21. CML Philadelphia chromosome – a short chromosome 22discovered at 1960 by Novel and Henderford First chromosomal
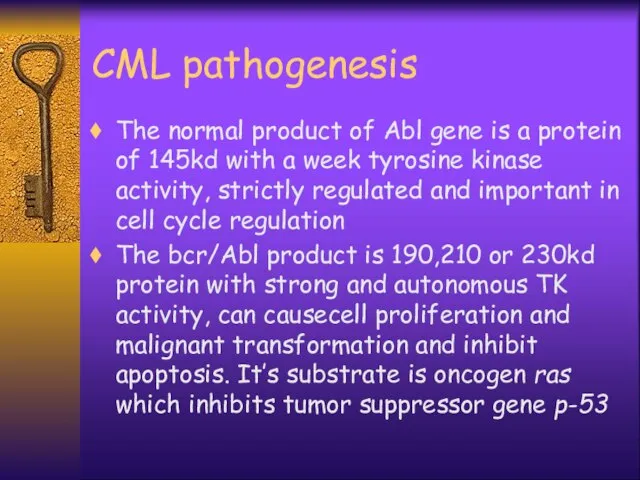
- 23. CML pathogenesis The normal product of Abl gene is a protein of 145kd with a week
- 24. Clinical features Most patients are asymptomatic at diagnosis Splenomegaly ± symptoms, anemia, hepatomegaly, purpura, constitutional symptoms
- 25. Laboratory Peripheral blood : leukocytosis with “left shift”, basophillia, eeosinophilia, thrombocytosis, anemia Bone marrow: myeloid (M:E>3:1),
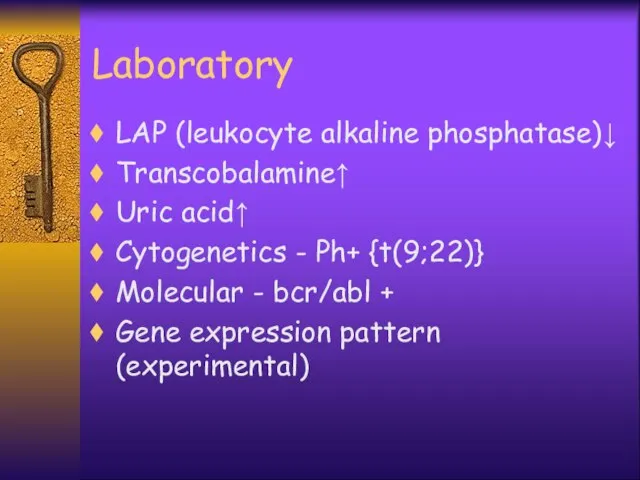
- 26. Laboratory LAP (leukocyte alkaline phosphatase)↓ Transcobalamine↑ Uric acid↑ Cytogenetics - Ph+ {t(9;22)} Molecular - bcr/abl +
- 27. Accelerated Phase ↑Leukocytosis under treatment ↑Basophilia (>20% basophils and eosinophils >10% blasts in peripheral blood >20%
- 28. BLAST CRISIS Developes in 75-80% of patients Median time from diagnosis 3-5 years constitutional symptoms, bone
- 29. TREATMENT Tyrosine kinase inhibitors - glyvec (imatinib mesylate), nilotinib, dasatinib etc., major cytogenetic and molecular responses
- 30. C L L A progressive accumulation of functionally incompetent mature lymphocytes 15-20% of all leukemias, M:F=1.7:1
- 31. C L L Frequent family history of CLL, other B-cell malignancies, autoimmune disorders No other risk
- 32. C L L Immunophenotyping: B-cell markers CD19, CD20, CD21, CD23, ; T-cell marker CD5 is a
- 33. Clinical Manifestations Autoimmune features - Coomb’s+ hemolytic anemia, ITP Recurrent infections - due to hypogammaglobulinemia Symptoms:
- 34. Laboratory Findings >5000 mature appearing lymphocytes Anemia, thrombocytopenia Bone marrow - infiltration by same lymphocytes, decrease
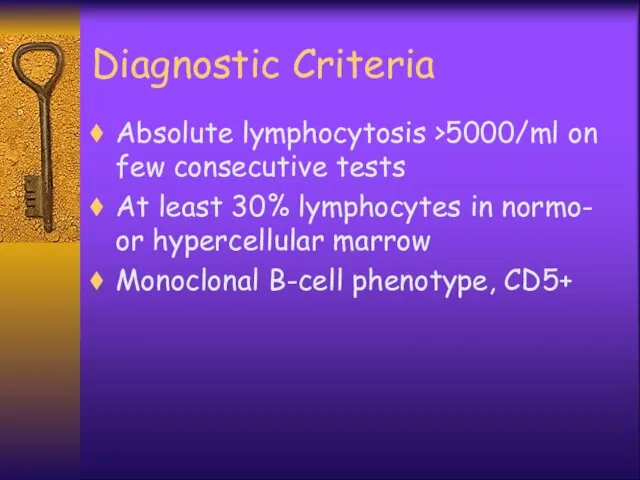
- 35. Diagnostic Criteria Absolute lymphocytosis >5000/ml on few consecutive tests At least 30% lymphocytes in normo- or
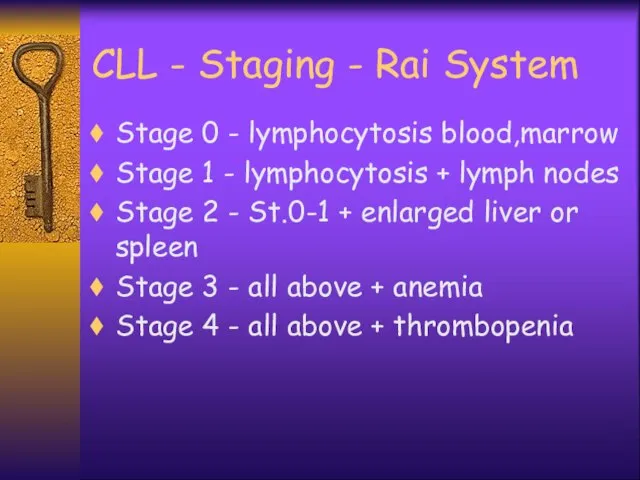
- 36. CLL - Staging - Rai System Stage 0 - lymphocytosis blood,marrow Stage 1 - lymphocytosis +
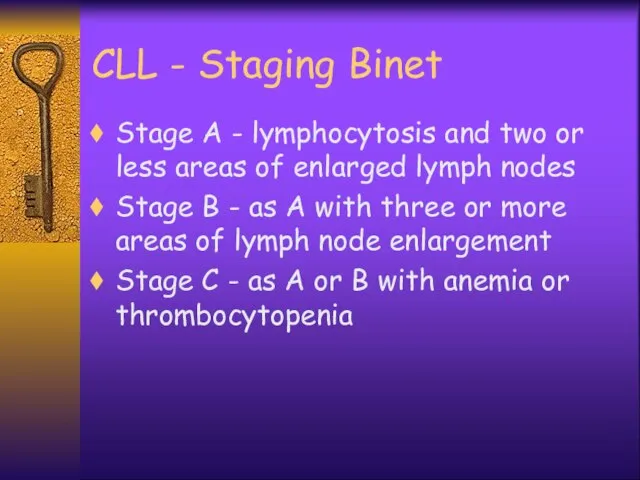
- 37. CLL - Staging Binet Stage A - lymphocytosis and two or less areas of enlarged lymph
- 38. CLL -Treatment Rai st. 0-2 or Binet st. A-B ⇒ observe every 3-6 months, treat if
- 39. Treatment Options Chemotherapy - steroids, alkylating agents ± steroids, purine analogues - fludarabine, combinations Monoclonal antibodies
- 40. CLL - Prognosis Extremely variable - some have progressive course and die within 2-3 years, some
- 41. Richter’s Syndrome In 3-5% the disease undergoes a transformation into aggressive lymphoma - diffuse large cell
- 43. Скачать презентацию





















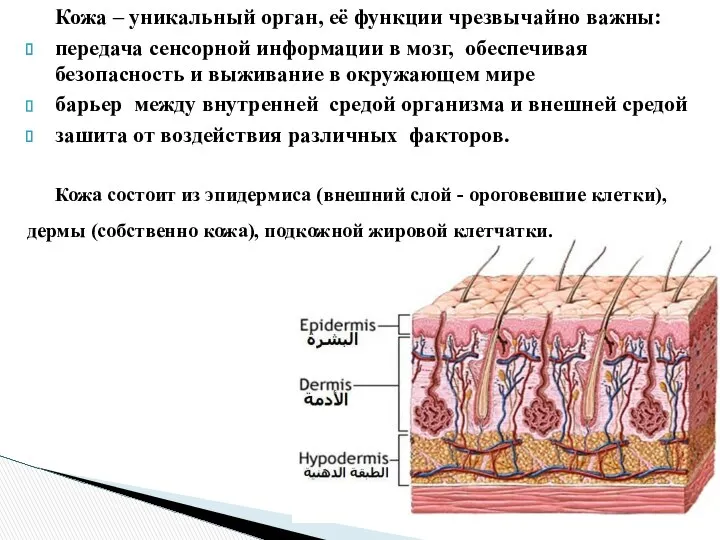 Кожа. Заболевания кожи
Кожа. Заболевания кожи Спирография. Понятие внешнего дыхания
Спирография. Понятие внешнего дыхания Противогрибковые и противовирусные лечебные средства
Противогрибковые и противовирусные лечебные средства Возрастные особенности роста и физического развития подростков (проверочные тесты )
Возрастные особенности роста и физического развития подростков (проверочные тесты ) Опасен ли целлюлит для здоровья
Опасен ли целлюлит для здоровья Симптоматология гепатитов, цирроза печени, холецистита, дискипезии желчевыводящих путей
Симптоматология гепатитов, цирроза печени, холецистита, дискипезии желчевыводящих путей Паразиты
Паразиты Истерия
Истерия Хронический пульпит
Хронический пульпит Влияние излучения, исходящего из сотового телефона на организм человека
Влияние излучения, исходящего из сотового телефона на организм человека Опухоли кроветворной ткани
Опухоли кроветворной ткани Эпидемиология и профилактика антропонозов с контактным механизмом передачи
Эпидемиология и профилактика антропонозов с контактным механизмом передачи Возрастные особенности суицидального поведения
Возрастные особенности суицидального поведения Жасқа дейінгі балардың психо-моторлы даму ерекшеліктері
Жасқа дейінгі балардың психо-моторлы даму ерекшеліктері Периферический и центральный отделы речевого аппарата
Периферический и центральный отделы речевого аппарата Чувства, эмоции
Чувства, эмоции Реанимация и интенсивная терапия острых отравлений. Особенности реанимационного пособия при несчастных случаях
Реанимация и интенсивная терапия острых отравлений. Особенности реанимационного пособия при несчастных случаях Первая помощь пострадавшим и её значение
Первая помощь пострадавшим и её значение Презентация по медицине Термические повреждения,ожоги, электротравмы
Презентация по медицине Термические повреждения,ожоги, электротравмы  Анонимные Наркоманы
Анонимные Наркоманы Выготский Лев Семёнович (1896—1934)
Выготский Лев Семёнович (1896—1934) Острые бактериальные воздушно-капельные инфекции
Острые бактериальные воздушно-капельные инфекции Эпилепсия
Эпилепсия Соматика Томаса Ханны и метод Фельденкрайза
Соматика Томаса Ханны и метод Фельденкрайза Мировые стандарты качества и безопасности продуктов питания
Мировые стандарты качества и безопасности продуктов питания Нейровизуализация в неврологии
Нейровизуализация в неврологии Новое слово в иммунологии! вторая жизнь сенсационного научного открытия прошлого века
Новое слово в иммунологии! вторая жизнь сенсационного научного открытия прошлого века Шаблоны для проведения имплантации зубов
Шаблоны для проведения имплантации зубов