Содержание
- 2. Portions of this presentation are based on non-malignant CNS tumor data collection rules adopted by the
- 3. Part I Rationale History Definition of Reportable Cases Casefinding Anticipated Impact on Registries
- 4. Rationale for Non-malignant CNS Tumor Surveillance and Registration Non-malignant CNS tumors cause disruption in normal function
- 5. History 1992 -1996 1992 Central Brain Tumor Registry of the United States (CBTRUS) formed to report
- 6. History 1998 BTWG forwarded four recommendations to the NCCCS NCCCS Accepted recommendations 1 and 2 Deferred
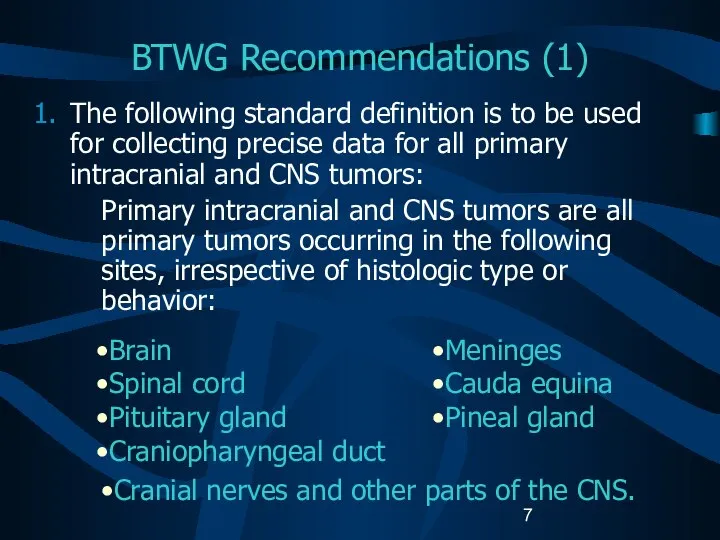
- 7. BTWG Recommendations (1) The following standard definition is to be used for collecting precise data for
- 8. BTWG Recommendations (2) Develop a standard site and histology definition for tabulating estimates of CNS tumors
- 9. BTWG Recommendations (3) Develop training for reporting and tabulating primary intracranial and CNS tumors, and develop
- 10. History 2000 International Classification of Diseases for Oncology 3rd Edition (ICD-O-3) and World Health Organization (WHO)
- 11. History 2001-2002 2001 NCCCS Accepted Recommendations 1 and 2 as completed. Reconvened the BTWG to work
- 12. Reportable Brain-Related Tumors (1) Public Law 107-260 requires reporting of brain-related tumors. The term “brain-related tumor”
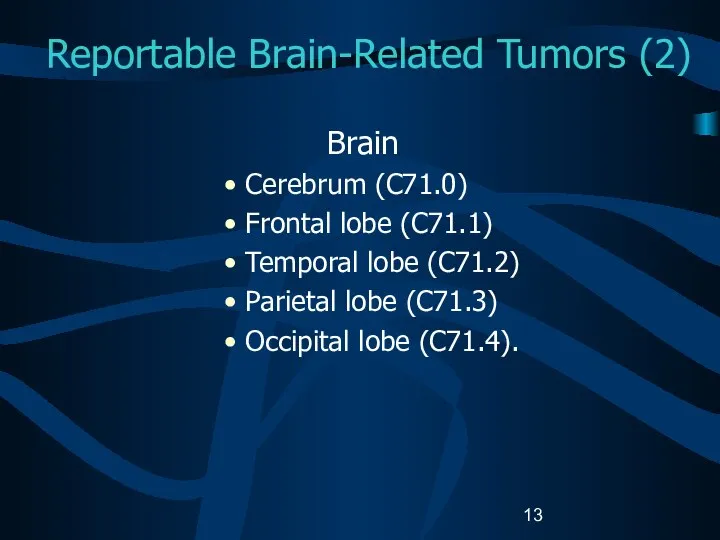
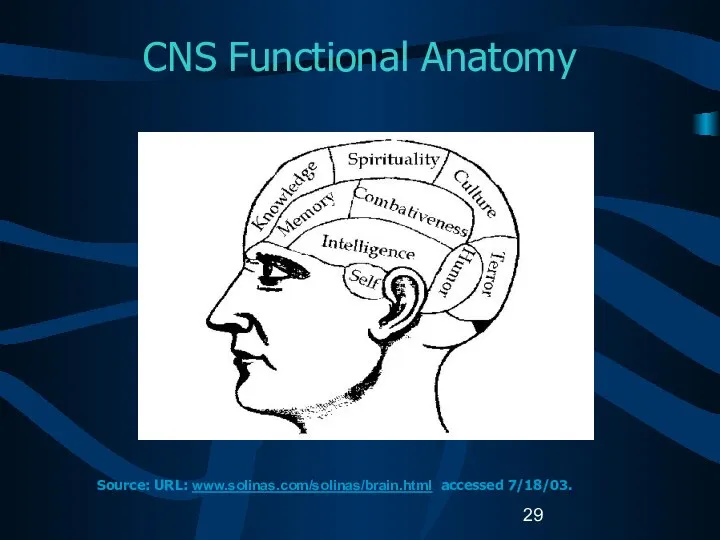
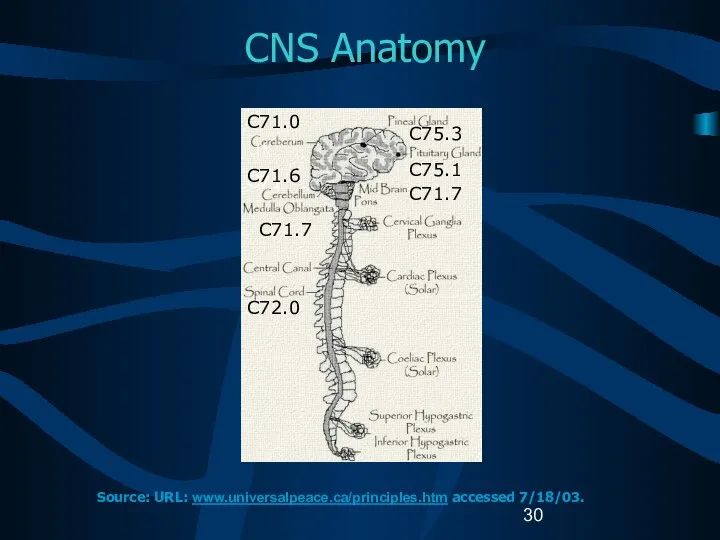
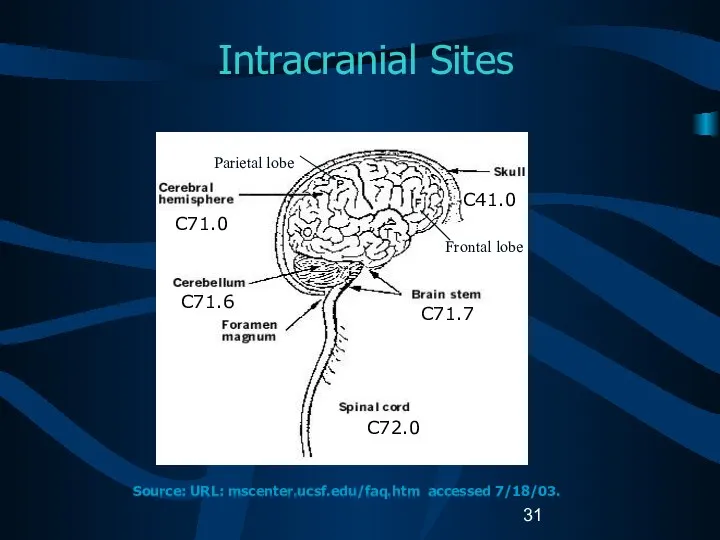
- 13. Reportable Brain-Related Tumors (2) Brain Cerebrum (C71.0) Frontal lobe (C71.1) Temporal lobe (C71.2) Parietal lobe (C71.3)
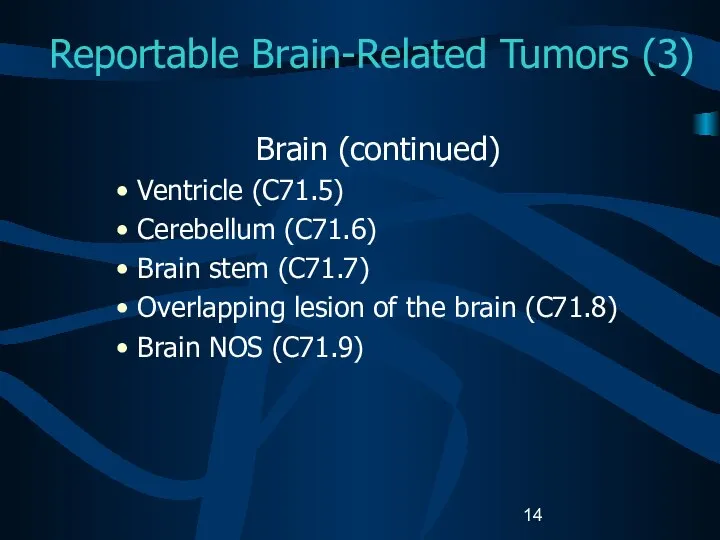
- 14. Reportable Brain-Related Tumors (3) Brain (continued) Ventricle (C71.5) Cerebellum (C71.6) Brain stem (C71.7) Overlapping lesion of
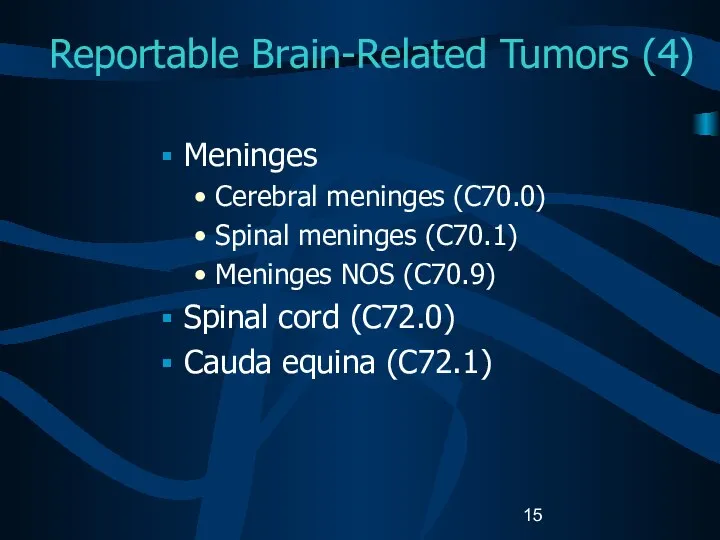
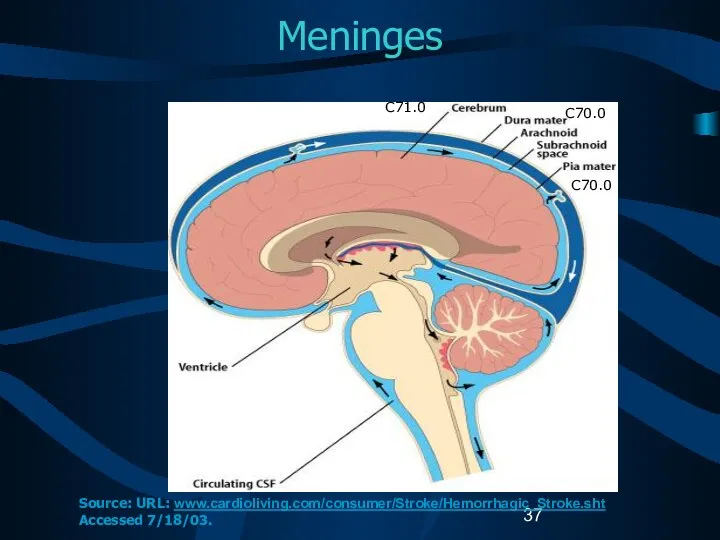
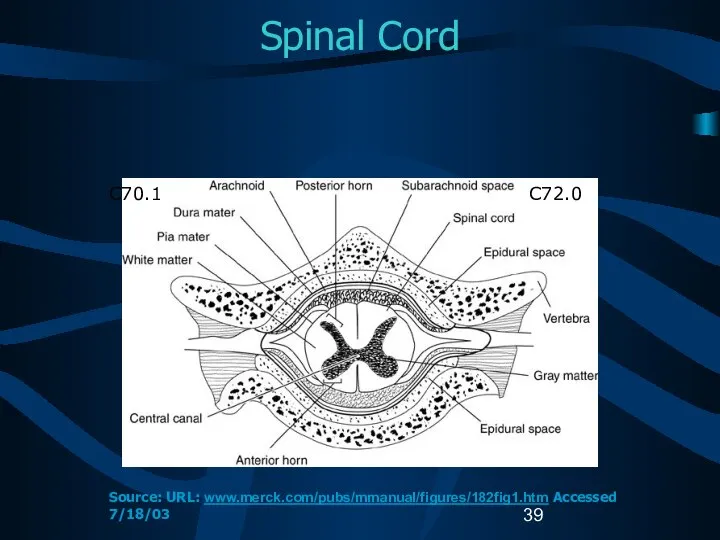
- 15. Reportable Brain-Related Tumors (4) Meninges Cerebral meninges (C70.0) Spinal meninges (C70.1) Meninges NOS (C70.9) Spinal cord
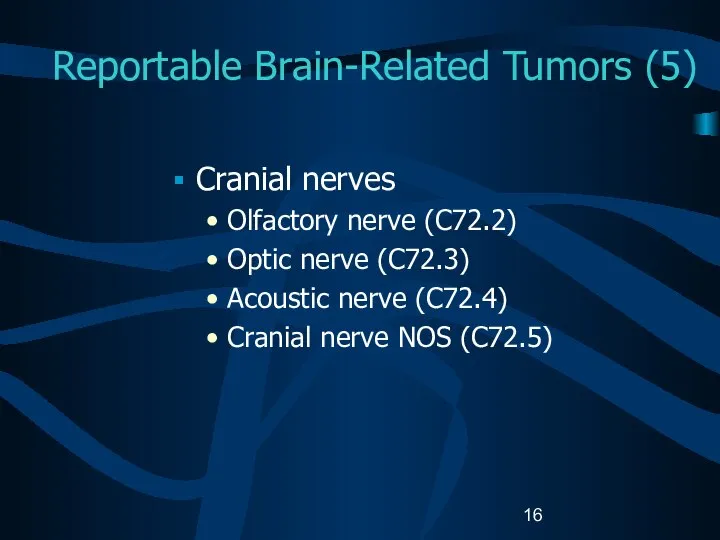
- 16. Reportable Brain-Related Tumors (5) Cranial nerves Olfactory nerve (C72.2) Optic nerve (C72.3) Acoustic nerve (C72.4) Cranial
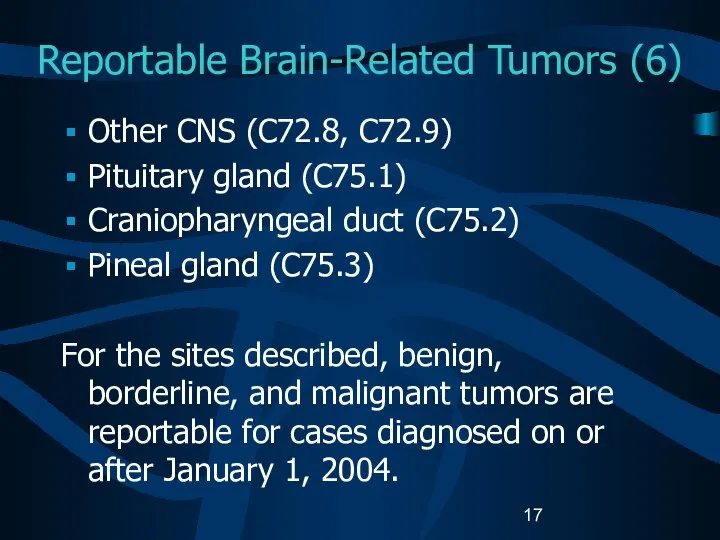
- 17. Reportable Brain-Related Tumors (6) Other CNS (C72.8, C72.9) Pituitary gland (C75.1) Craniopharyngeal duct (C75.2) Pineal gland
- 18. History 2003 2003 SEER-supported registries and COC-approved hospital cancer registries will also report non-malignant CNS tumors
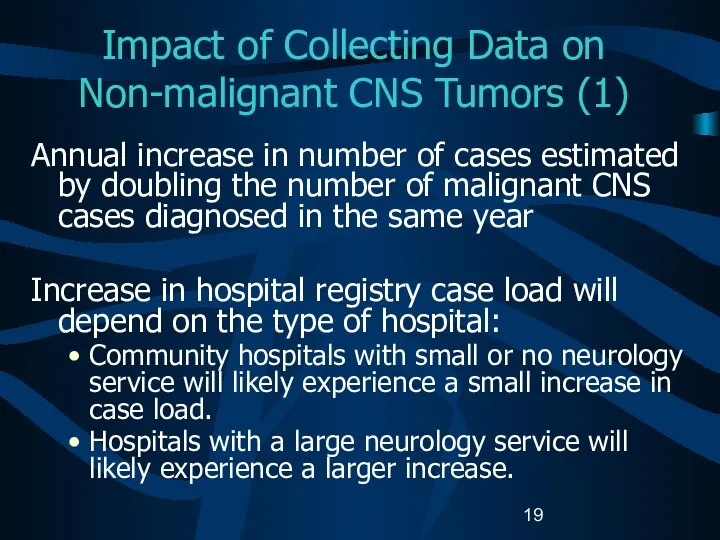
- 19. Impact of Collecting Data on Non-malignant CNS Tumors (1) Annual increase in number of cases estimated
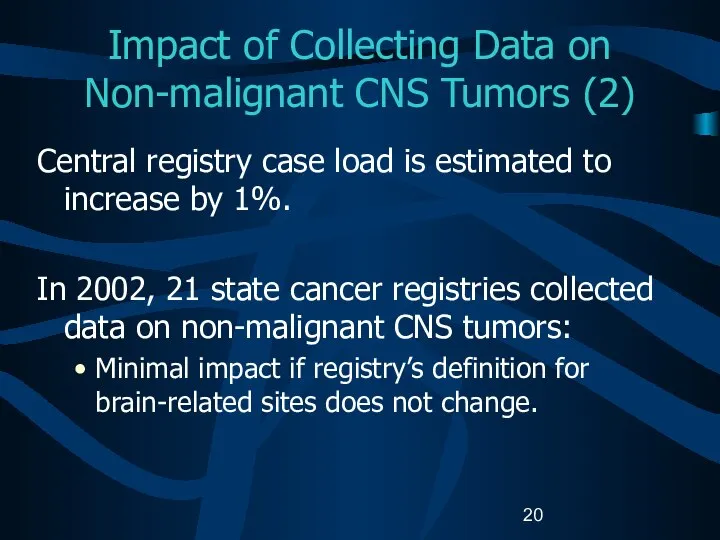
- 20. Impact of Collecting Data on Non-malignant CNS Tumors (2) Central registry case load is estimated to
- 21. Impact of Collecting Data on Non-malignant CNS Tumors (3) Central registries adding non-malignant CNS tumors to
- 22. Impact of Collecting Data on Non-malignant CNS Tumors (4) All cancer registries must: Have the same
- 23. Case-finding (1) Additional or expanded case-finding mechanisms: Pathology Radiology Treatment facilities: Radiation oncology centers and departments
- 24. Case-finding (2) Disease indices Surgery logs Diagnostic imaging Radiation oncology Neurology clinics Medical oncology Autopsy reports.
- 25. Case-finding Sources Free-standing radiation therapy centers Free-standing Magnetic Resonance Imaging (MRI) centers Free-standing gamma or cyber
- 26. ICD-9-CM Codes for Case-finding
- 27. Unusual and Ambiguous Terminology If the final pathologic diagnosis is a CNS “neoplasm” or “mass”, an
- 28. Part II CNS Anatomy and Function Histologies and Primary Sites Grading Systems and Coding Grade
- 29. CNS Functional Anatomy Source: URL: www.solinas.com/solinas/brain.html accessed 7/18/03.
- 30. CNS Anatomy C71 C71.6 C71.7 C72.0 C71.0 C75.3 C75.1 C71.7 Source: URL: www.universalpeace.ca/principles.htm accessed 7/18/03.
- 31. Intracranial Sites C71.0 C71.6 C41.0 C71.7 C72.0 Source: URL: mscenter.ucsf.edu/faq.htm accessed 7/18/03. Parietal lobe Frontal lobe
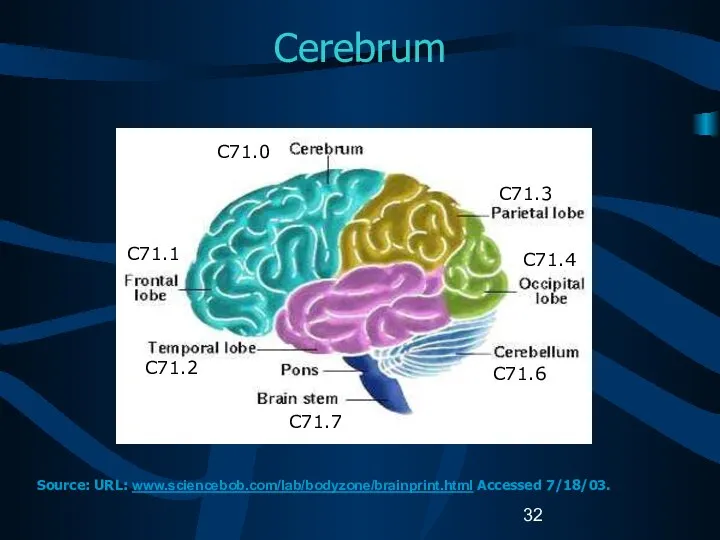
- 32. Cerebrum C71.1 C71.2 C71.7 C71.3 C71.4 C71.6 C71.0 Source: URL: www.sciencebob.com/lab/bodyzone/brainprint.html Accessed 7/18/03.
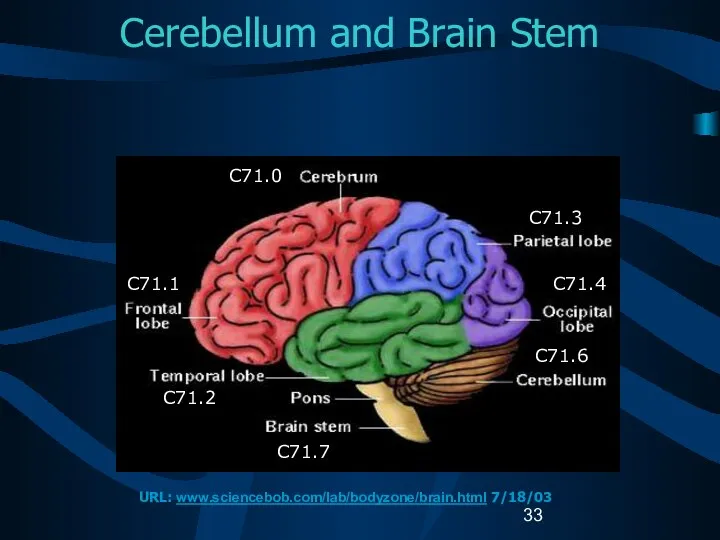
- 33. Cerebellum and Brain Stem C71.0 C71.1 C71.2 C71.7 C71.3 C71.4 C71.6 URL: www.sciencebob.com/lab/bodyzone/brain.html 7/18/03
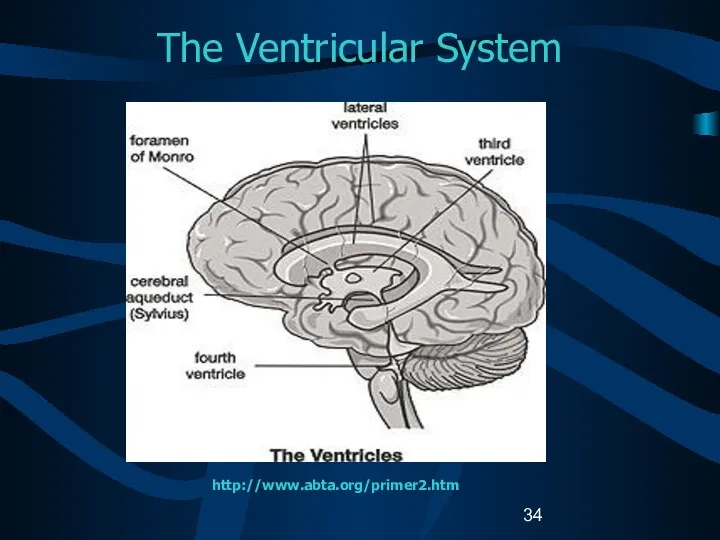
- 34. The Ventricular System http://www.abta.org/primer2.htm
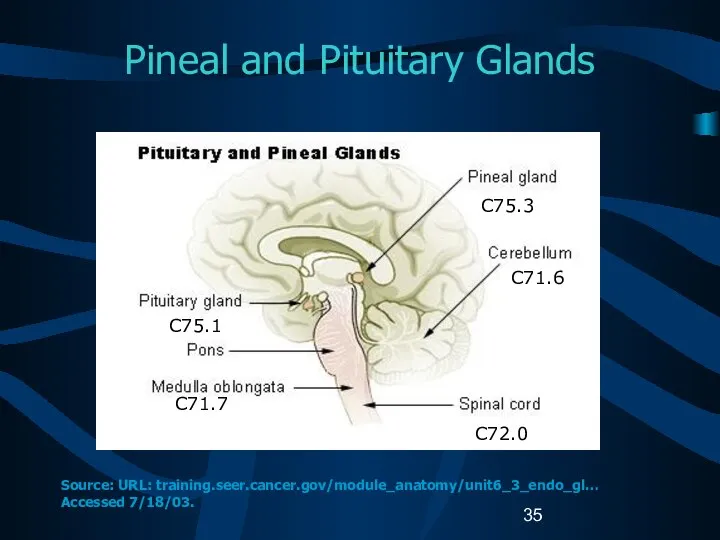
- 35. Pineal and Pituitary Glands C75.1 C71.7 C75.3 C71.6 C72.0 Source: URL: training.seer.cancer.gov/module_anatomy/unit6_3_endo_gl… Accessed 7/18/03.
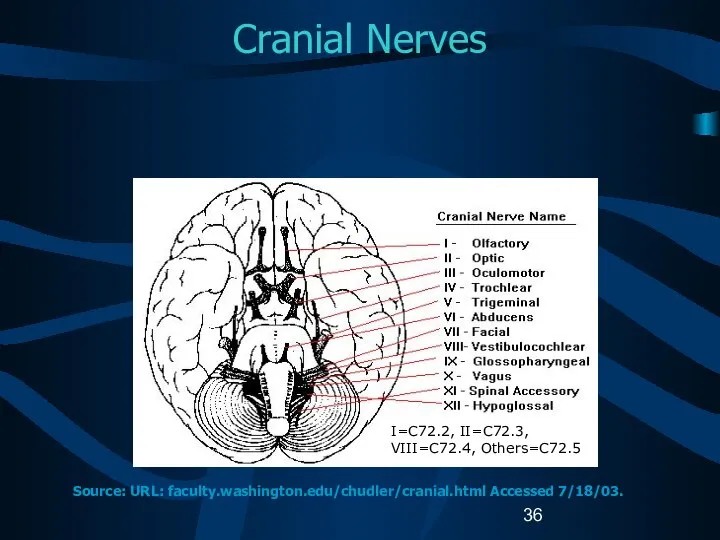
- 36. Cranial Nerves I=C72.2, II=C72.3, VIII=C72.4, Others=C72.5 Source: URL: faculty.washington.edu/chudler/cranial.html Accessed 7/18/03.
- 37. Meninges C71.0 C70.0 C70.0 Source: URL: www.cardioliving.com/consumer/Stroke/Hemorrhagic_Stroke.sht Accessed 7/18/03.
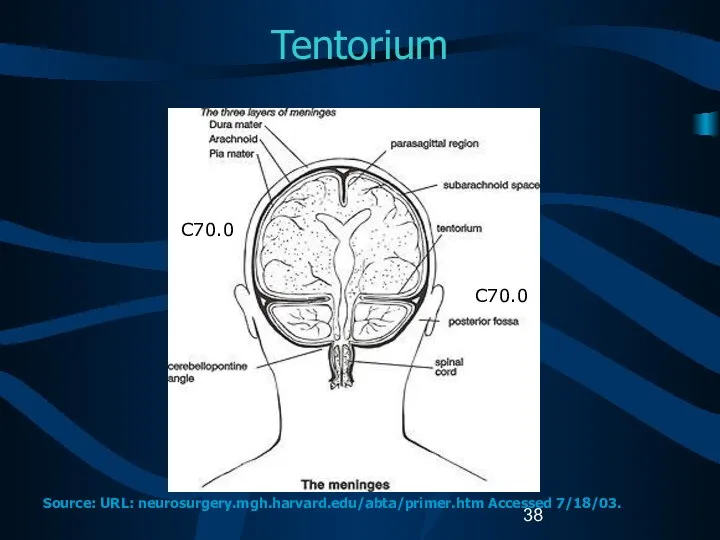
- 38. Tentorium C70.0 C70.0 Source: URL: neurosurgery.mgh.harvard.edu/abta/primer.htm Accessed 7/18/03.
- 39. Spinal Cord C72.0 C70.1 Source: URL: www.merck.com/pubs/mmanual/figures/182fig1.htm Accessed 7/18/03
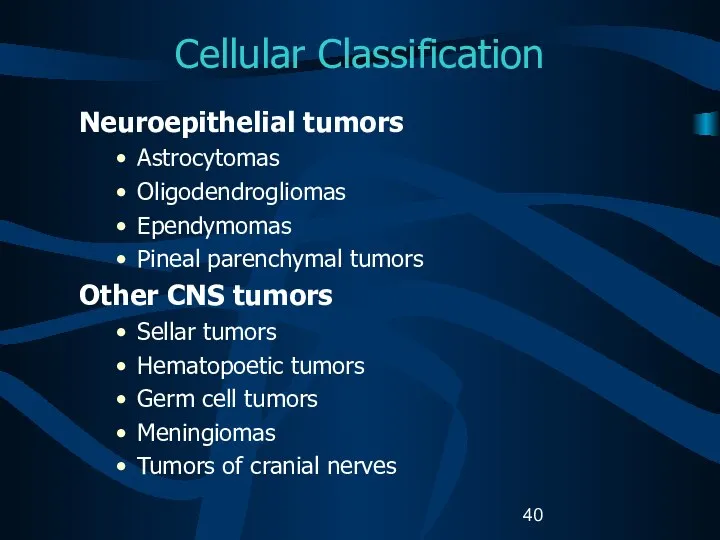
- 40. Cellular Classification Neuroepithelial tumors Astrocytomas Oligodendrogliomas Ependymomas Pineal parenchymal tumors Other CNS tumors Sellar tumors Hematopoetic
- 41. Glial Tumors (1) Glial tissue: supportive tissue of brain made up of astrocytes and oligodendrocytes Glial
- 42. Glial Tumors (2) Astrocytic tumors Noninfiltrating Juvenile pilocytic (M9421) Subependymal (M9383) Infiltrating Well-differentiated mildly and moderately
- 43. Glial Tumors (3) Ependymal tumors Myxopapillary and well-differentiated ependymomas (M9394) Anaplastic ependymomas (M9392) Ependymoblastomas (M9392) Oligodendroglial
- 44. Glial Tumors (4) Mixed tumors Mixed astrocytoma-ependymomas Mixed astrocytoma-oligodendrogliomas Mixed astrocytoma-ependymoma-oligodendrogliomas Other gliomas Ganglioneuromas (M9490) Optic
- 45. Non-Glial Tumors (1) Pineal region tumors Parenchymal tumors Pineocytomas (M9361) Pineoblastomas (M9362) Pineal astrocytomas (M9400) Germ
- 46. Non-Glial Tumors (2) Meningiomas Meningioma: Benign (M953_) Malignant meningiomas Anaplastic meningioma Hemangiopericytoma (M9150) Papillary meningioma (M9538)
- 47. Other CNS Tumors (1) Craniopharyngiomas (M9350) Rathke pouch tumors Chordomas (M9370) Schwannomas (M9560) Acoustic schwannomas/neuromas
- 48. Other CNS Tumors (2) Embryonal tumors Retinoblastomas (M9510) Primitive neuroectodermal tumors (PNETs) Meduloblastomas (M9470) Neuroblastomas (M9500)
- 49. Other CNS Tumors (3) Lymphomas (M9590) Arise from Indigenous brain histiocytes (microglia) Rare lymphocytes in meninges
- 50. Other CNS Tumors (4) Cysts and tumor-like lesions Reportable Dermoid cysts (M9084) Granular cell tumors (M9580)
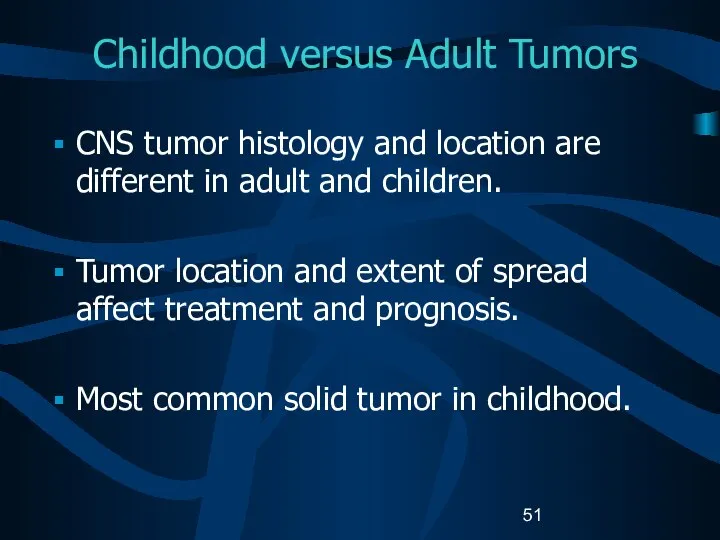
- 51. Childhood versus Adult Tumors CNS tumor histology and location are different in adult and children. Tumor
- 52. Childhood Brain Tumors Meduloblastomas are the most common CNS histology in children. 50% are infratentorial. Common
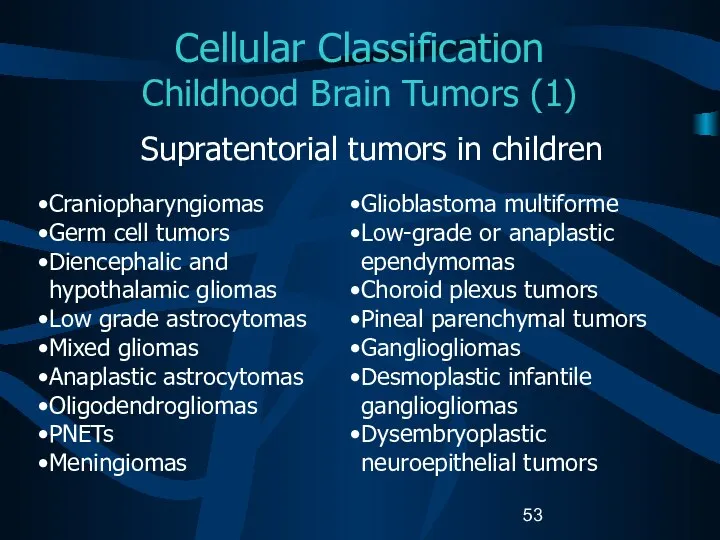
- 53. Cellular Classification Childhood Brain Tumors (1) Supratentorial tumors in children Craniopharyngiomas Germ cell tumors Diencephalic and
- 54. Cellular Classification Childhood Brain Tumors (2) The histopathology of childhood spinal tumors is the same as
- 55. Cellular Classification Childhood CNS Tumors Cause of childhood CNS tumors remains unknown. American Academy of Pediatrics
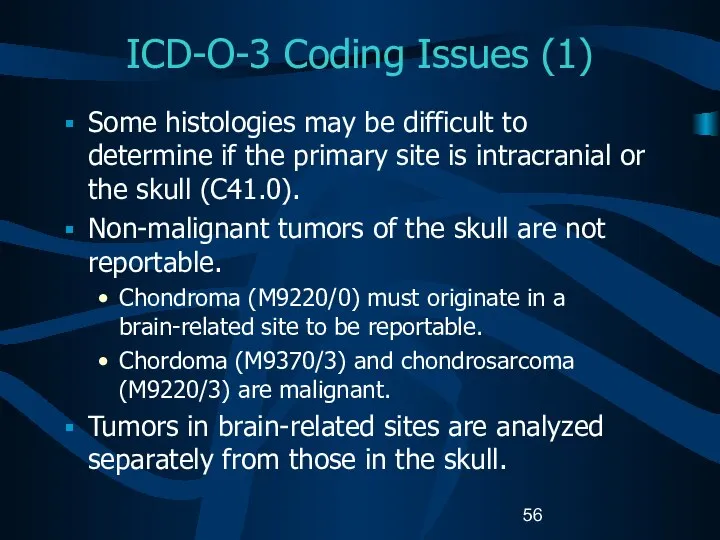
- 56. ICD-O-3 Coding Issues (1) Some histologies may be difficult to determine if the primary site is
- 57. ICD-O-3 Coding Issues (2) Continue to assign histology code M9421/3 to pilocytic astrocytoma. When the primary
- 58. Grade for CNS Tumors Sixth digit of ICD-O-3 histology code Describes tumor differentiation or grade. Is
- 59. WHO Grade (1) WHO grade coded in Collaborative Stage data field: Site-specific factor 1 for Brain.
- 60. WHO Grade (2) Grade II Relatively slow growing Sometimes recur as higher grade tumors May be
- 61. Kernohan Grade Defines progressive malignancy for astrocytoma Grade 1: benign astrocytomas Grade 2: low-grade astrocytomas Grade
- 62. St. Anne-Mayo Grade (1) Used for astrocytomas. Uses four morphologic criteria: Nuclear atypia Mitosis Endothelial proliferation
- 63. St. Anne-Mayo Grade (2) Grade 1: No criteria Grade 2: One criterion, usually nuclear atypia Grade
- 64. Grade for CNS Tumors Do not record WHO grade, Kernohan grade, or St. Anne/Mayo grade in
- 65. Part III Laterality Multiple Primaries Malignant Transformation Sequence Numbers Date of Diagnosis
- 66. Determining Multiple Primaries: Laterality Brain is not a paired organ. Laterality collected on both non-malignant and
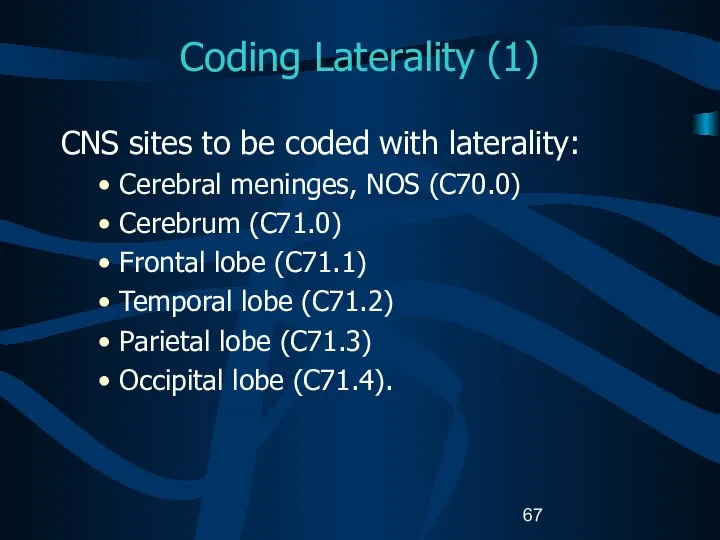
- 67. Coding Laterality (1) CNS sites to be coded with laterality: Cerebral meninges, NOS (C70.0) Cerebrum (C71.0)
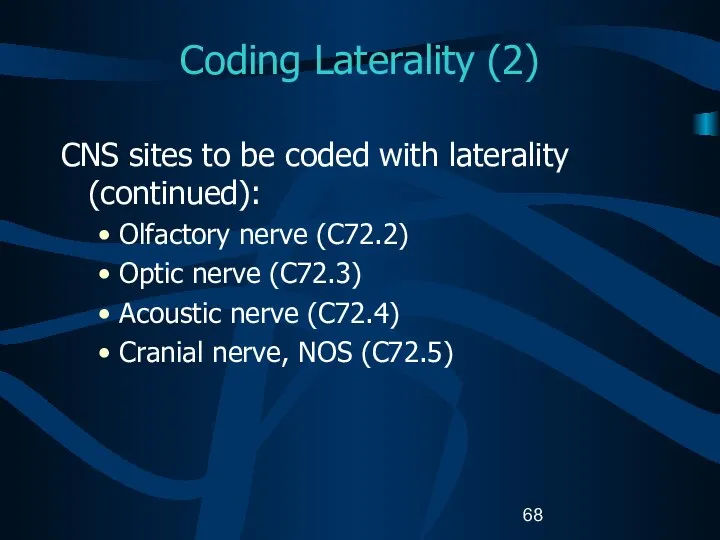
- 68. Coding Laterality (2) CNS sites to be coded with laterality (continued): Olfactory nerve (C72.2) Optic nerve
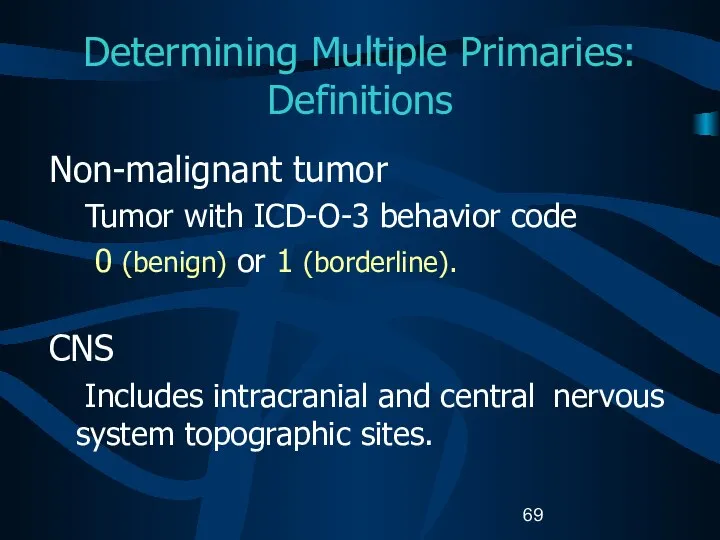
- 69. Determining Multiple Primaries: Definitions Non-malignant tumor Tumor with ICD-O-3 behavior code 0 (benign) or 1 (borderline).
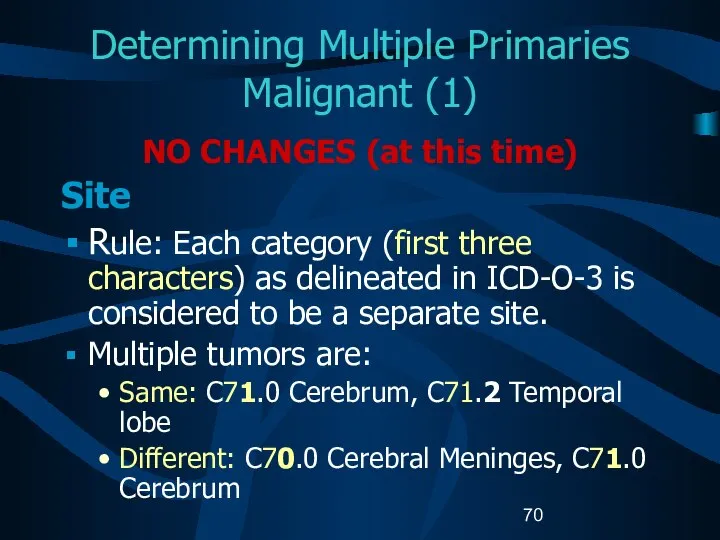
- 70. Determining Multiple Primaries Malignant (1) NO CHANGES (at this time) Site Rule: Each category (first three
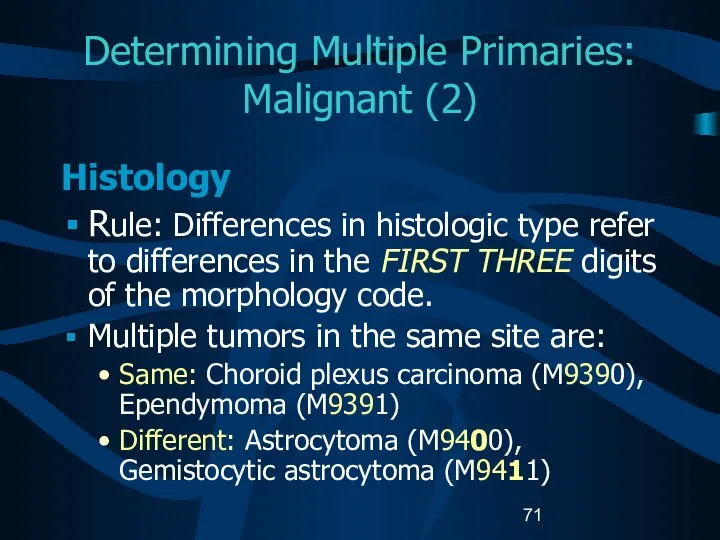
- 71. Determining Multiple Primaries: Malignant (2) Histology Rule: Differences in histologic type refer to differences in the
- 72. Determining Multiple Primaries Non-malignant (1) NEW RULES Site Rule: Each sub-site (fourth-digit level) as delineated in
- 73. Determining Multiple Primaries Non-malignant (2) Site (cont) EXCEPT NOS (C_ _.9) with specific four-digit site code
- 74. Determining Multiple Primaries Non-malignant (3) Site (cont) Laterality: For non-malignant cases only If multiple tumors of
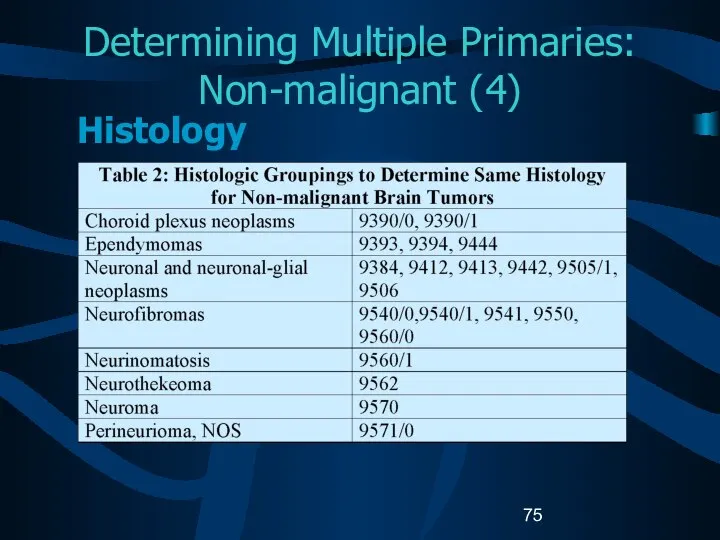
- 75. Determining Multiple Primaries: Non-malignant (4) Histology
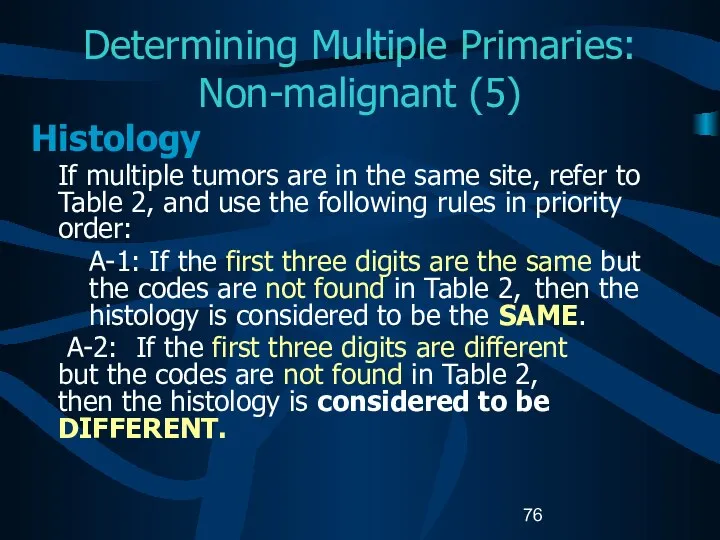
- 76. Determining Multiple Primaries: Non-malignant (5) Histology If multiple tumors are in the same site, refer to
- 77. Determining Multiple Primaries: Non-malignant (6) Histology (cont.) B. If all histologies are listed in the same
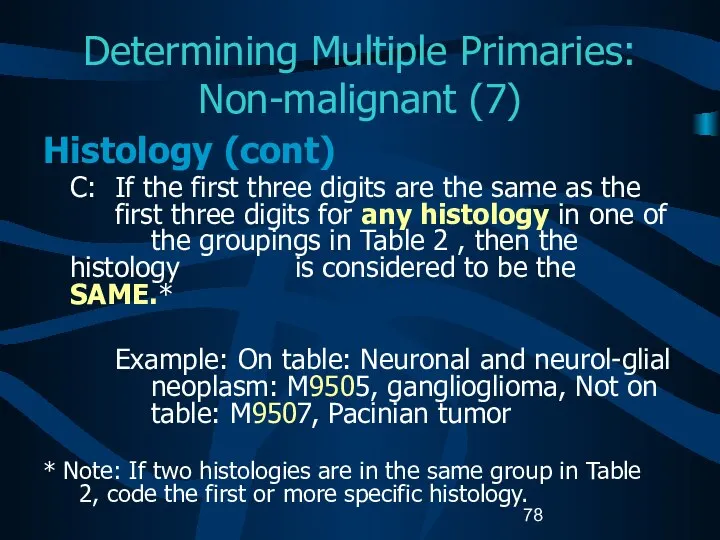
- 78. Determining Multiple Primaries: Non-malignant (7) Histology (cont) C: If the first three digits are the same
- 79. Determining Multiple Primaries: Non-malignant (8) Histology (cont) D: If the first three digits are the same
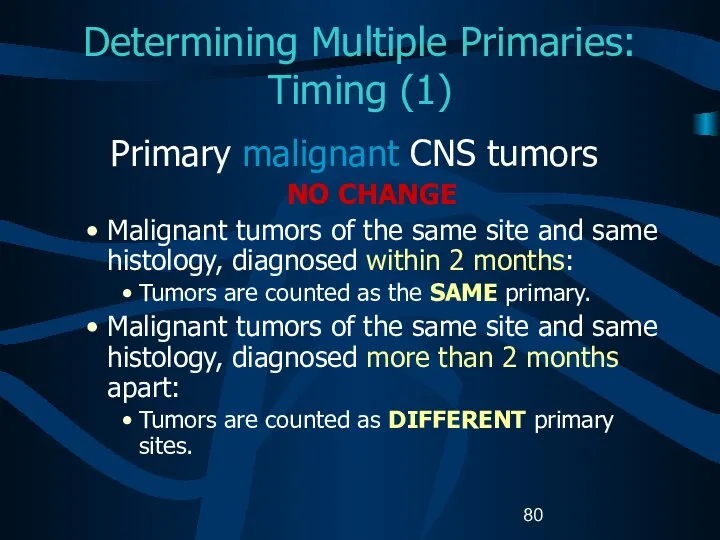
- 80. Determining Multiple Primaries: Timing (1) Primary malignant CNS tumors NO CHANGE Malignant tumors of the same
- 81. Determining Multiple Primaries: Timing (2) Primary non-malignant CNS tumors NEW No timing rule If a new
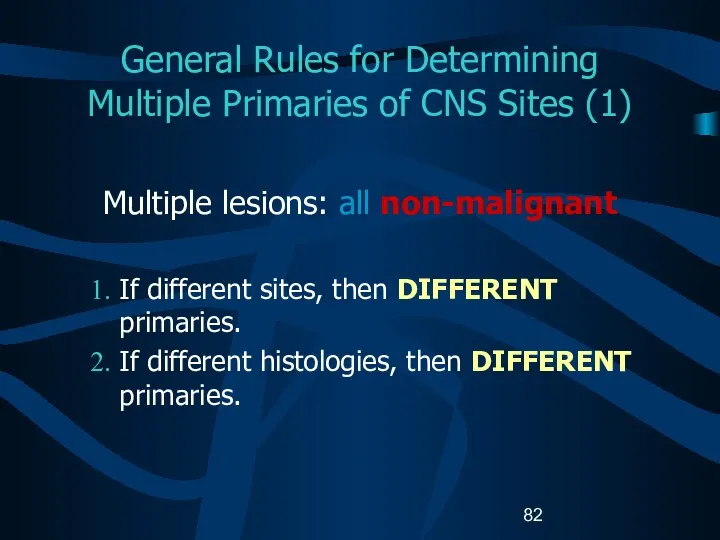
- 82. General Rules for Determining Multiple Primaries of CNS Sites (1) Multiple lesions: all non-malignant If different
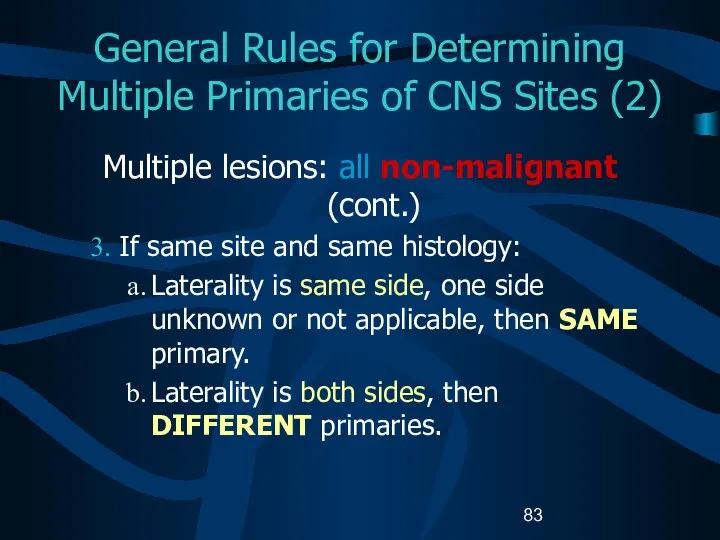
- 83. General Rules for Determining Multiple Primaries of CNS Sites (2) Multiple lesions: all non-malignant (cont.) If
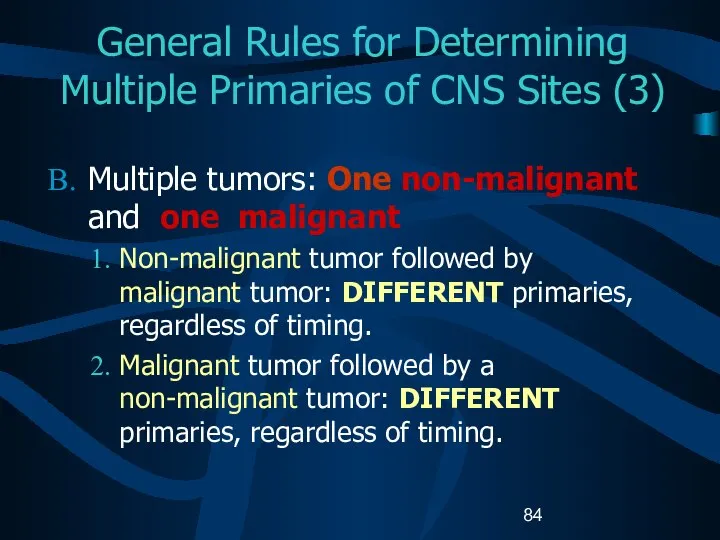
- 84. General Rules for Determining Multiple Primaries of CNS Sites (3) Multiple tumors: One non-malignant and one
- 85. Histologic Transformation (1) Histologic transformation or progression to a higher grade: Determined by pathological review. Final
- 86. Histologic Transformation (2) If a malignant CNS tumor recurs (transforms) as a higher grade tumor, SAME
- 87. Histologic Transformation (3) Transformation of a non-malignant tumor to a malignant tumor is rare. Malignant transformations
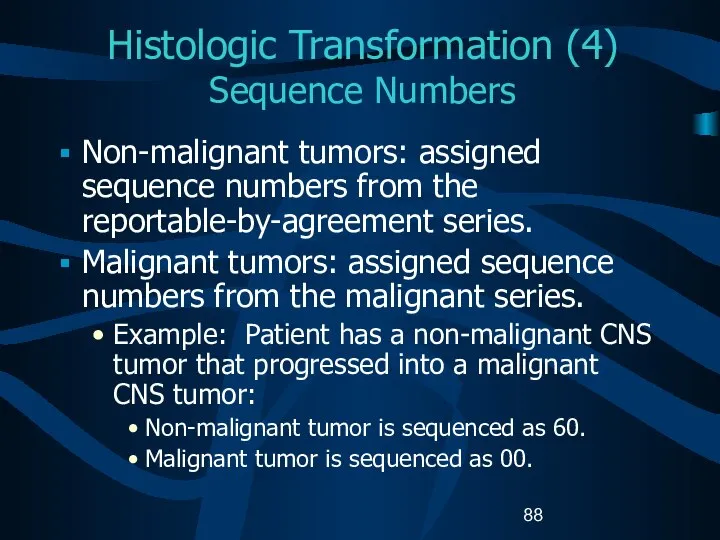
- 88. Histologic Transformation (4) Sequence Numbers Non-malignant tumors: assigned sequence numbers from the reportable-by-agreement series. Malignant tumors:
- 89. Histologic Transformation (5) Date of Diagnosis Non-malignant tumors: First date that a medical practitioner diagnosed the
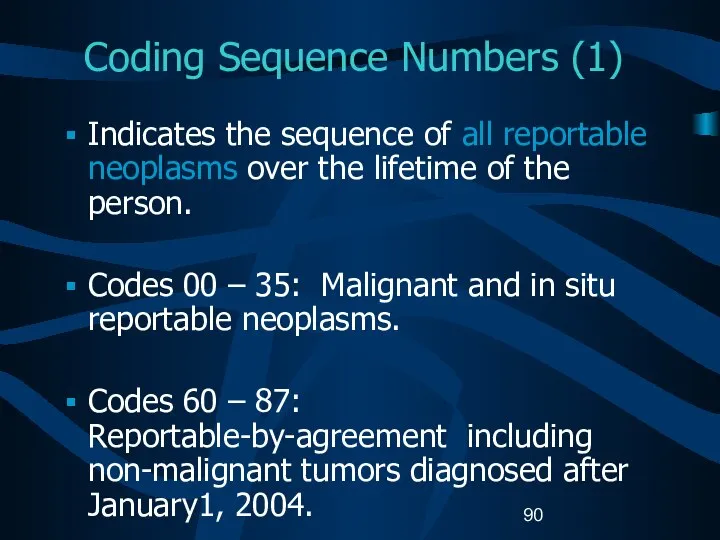
- 90. Coding Sequence Numbers (1) Indicates the sequence of all reportable neoplasms over the lifetime of the
- 91. Coding Sequence Numbers (2) Reportable-by-agreement neoplasms are defined by each facility and/or central cancer registry: Non-malignant
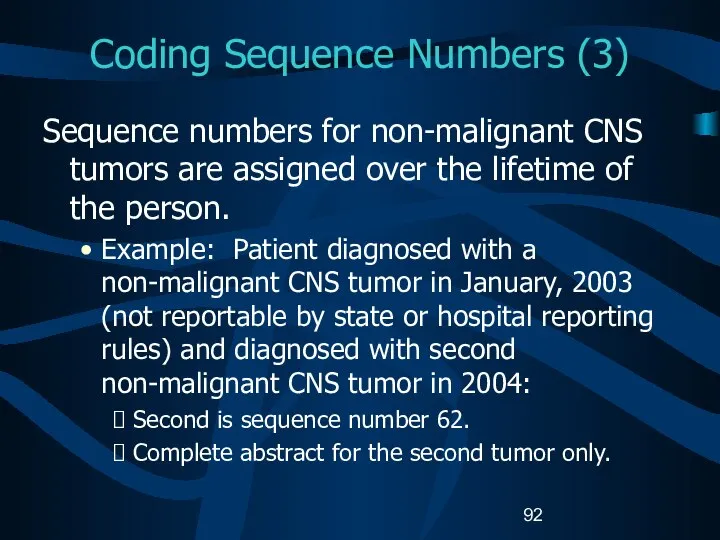
- 92. Coding Sequence Numbers (3) Sequence numbers for non-malignant CNS tumors are assigned over the lifetime of
- 93. Assigning Diagnosis Date Rules for assigning diagnosis date are the same for malignant and non-malignant tumors.
- 94. Part IV Staging Risk Factors Genetic Syndromes Diagnostic Tools Treatment Edits Data Analysis
- 95. Collaborative Stage (CS) A computer algorithm uses the collaborative stage (CS) data fields to calculate site-specific
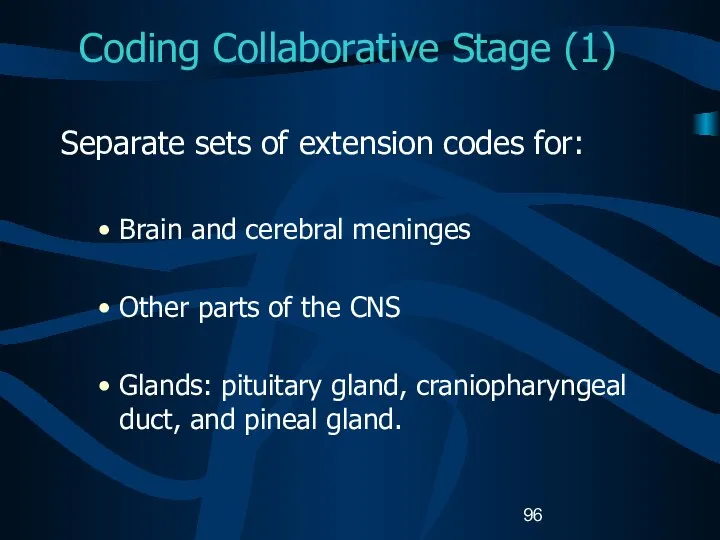
- 96. Coding Collaborative Stage (1) Separate sets of extension codes for: Brain and cerebral meninges Other parts
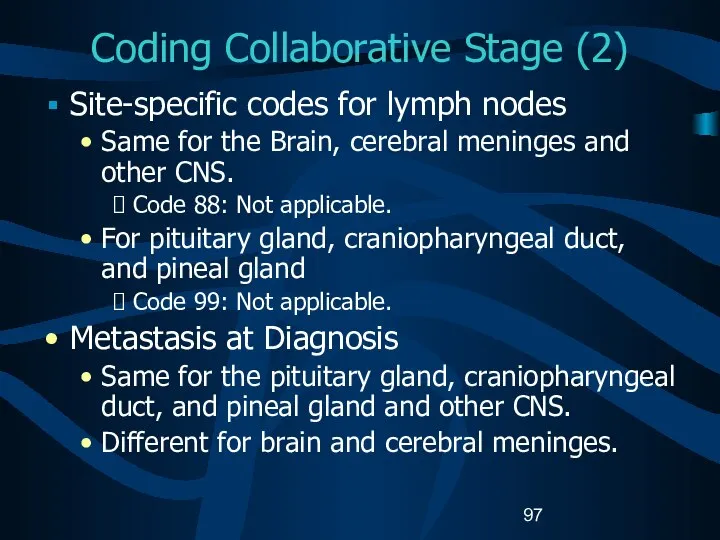
- 97. Coding Collaborative Stage (2) Site-specific codes for lymph nodes Same for the Brain, cerebral meninges and
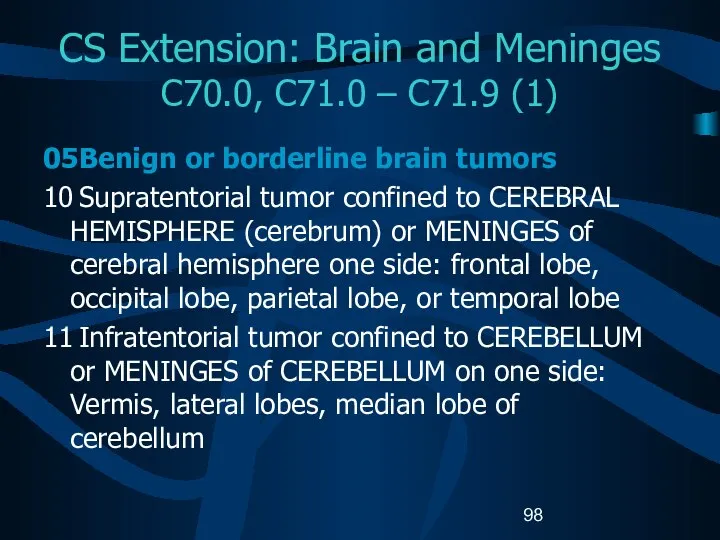
- 98. CS Extension: Brain and Meninges C70.0, C71.0 – C71.9 (1) 05 Benign or borderline brain tumors
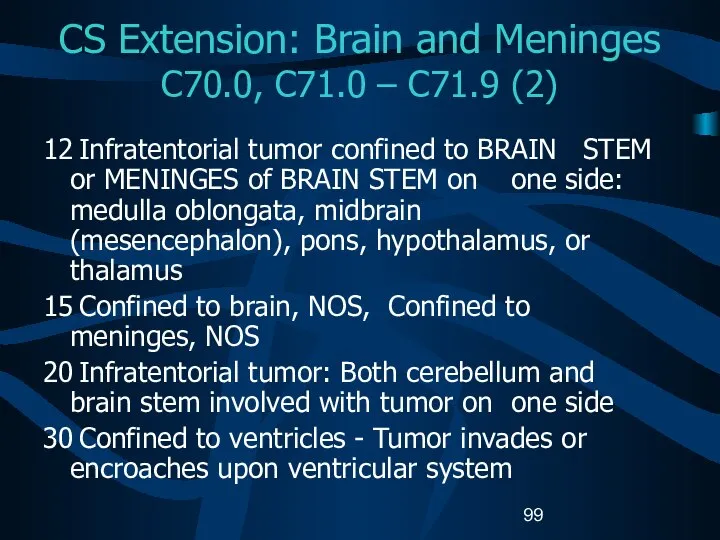
- 99. CS Extension: Brain and Meninges C70.0, C71.0 – C71.9 (2) 12 Infratentorial tumor confined to BRAIN
- 100. CS Extension: Brain and Meninges C70.0, C71.0 – C71.9 (3) 40 Tumor crosses the midline: involves
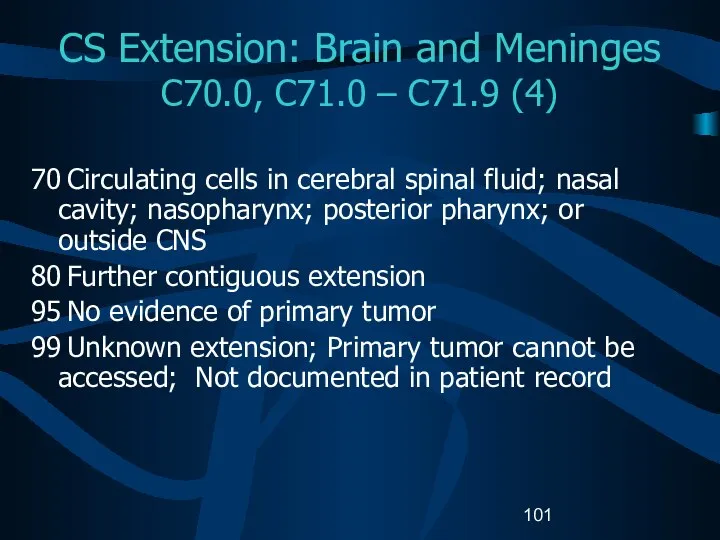
- 101. CS Extension: Brain and Meninges C70.0, C71.0 – C71.9 (4) 70 Circulating cells in cerebral spinal
- 102. CS Extension: Other CNS C70.1-9, C72.0–C72.9 (1) Spinal meninges, meninges NOS Spinal cord Caudia equina Olfactory,
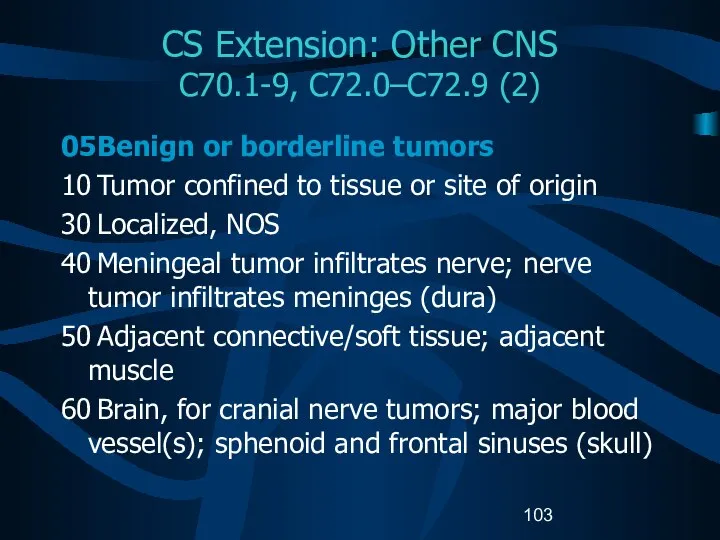
- 103. CS Extension: Other CNS C70.1-9, C72.0–C72.9 (2) 05 Benign or borderline tumors 10 Tumor confined to
- 104. CS Extension: Other CNS C70.1-9, C72.0–C72.9 (3) 70 Brain except for cranial nerve tumors; bone, other
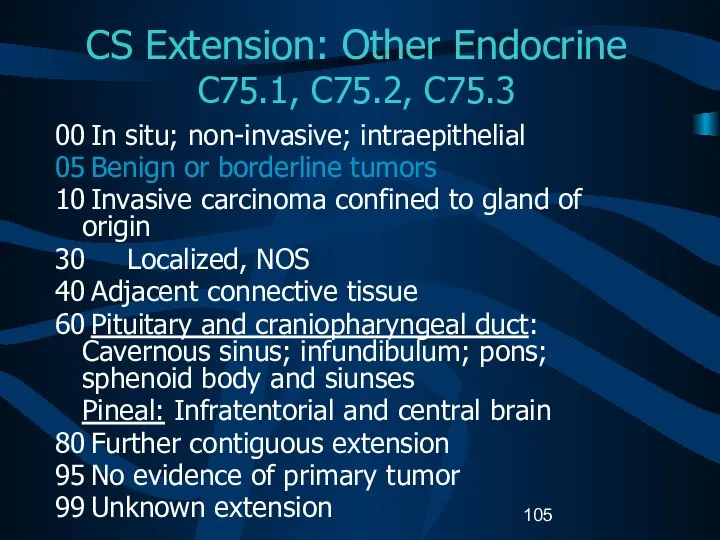
- 105. CS Extension: Other Endocrine C75.1, C75.2, C75.3 00 In situ; non-invasive; intraepithelial 05 Benign or borderline
- 106. CS Lymph Nodes Describes tumor involvement of regional lymph nodes. Code for CS Lymph Nodes is
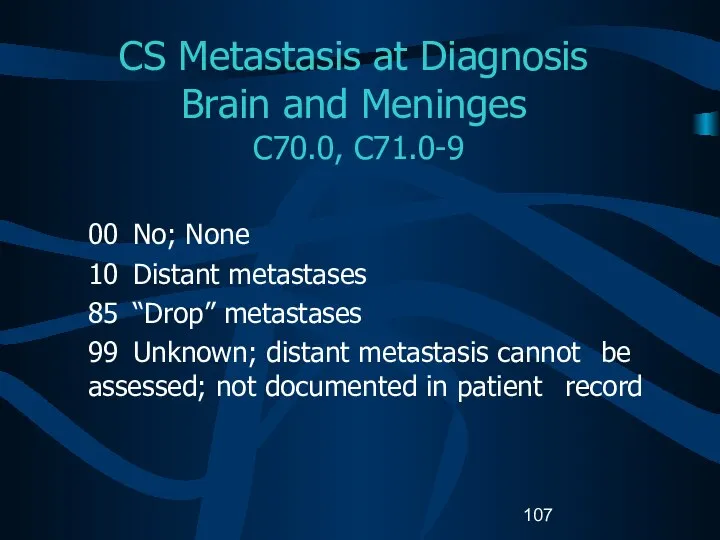
- 107. CS Metastasis at Diagnosis Brain and Meninges C70.0, C71.0-9 00 No; None 10 Distant metastases 85
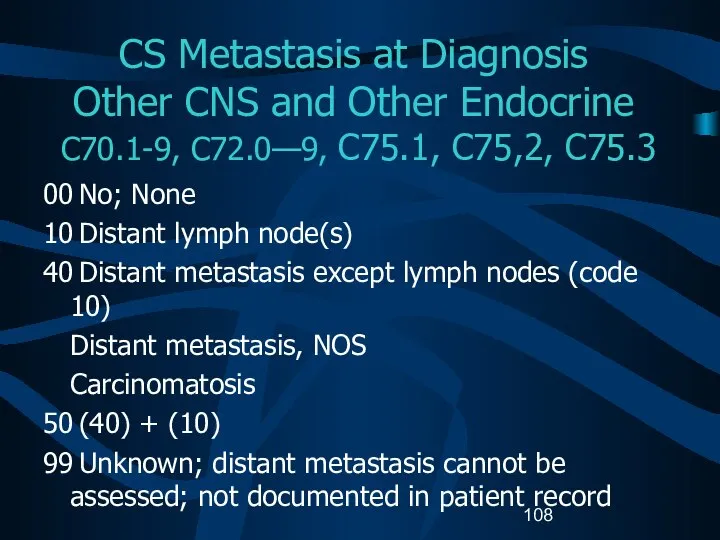
- 108. CS Metastasis at Diagnosis Other CNS and Other Endocrine C70.1-9, C72.0—9, C75.1, C75,2, C75.3 00 No;
- 109. CS Site-specific Factor 1 (1) C70.0-C70.9, C71.0-C71.9, C72.0-C72.9 010 WHO Grade I 020 WHO Grade II
- 110. CS Site-specific Factor 1 (2) C70.0-C70.9, C71.0-C71.9, C72.0-C72.9 C75.1- C75.3 Code the WHO grade for CNS
- 111. Possible Risk Factors Genetic predispositions for the development of brain tumors have been identified. Population-based studies
- 112. Possible Risk Factors Epstein-Barr virus in the DNA of primary lymphoma suggests a viral etiology for
- 113. Genetic Syndromes Genetic syndromes associated with multiple CNS tumors are: Neurofibromatosis I (von Recklinghausen’s disease) Neurofibromatosis
- 114. Diagnostic Tools – Physical Exam Neurological examination Eye movements Vision Hearing Reflexes Balance and coordination Sense
- 115. Diagnostic Tools: Radiology Computerized tomography (CT) scan Magnetic resonance imaging (MRI) Positron emission tomography (PET) Single
- 116. Diagnostic Tools: Laboratory tests Audiometry Electroencephalogram (EEG) Endocrine evaluation Evoked potentials Lumbar puncture Myelogram Perimetry
- 117. Diagnostic Tools Needle biopsy Needle inserted through a burr hole and tissue extracted for tissue diagnosis.
- 118. College of American Pathologist (CAP) Protocols Site-specific checklists Required to be completed in the health record
- 119. Brain and Spinal Cord CAP Protocols (1) Macroscopic Specimen type Specimen size Tumor site Tumor size
- 120. Brain and Spinal Cord CAP Protocols Microscopic Histologic type Histologic grade Margins Additional studies* Additional pathologic
- 121. Treatment (1) Watchful waiting Surgery Radiation Chemotherapy Hormonal therapy Immunotherapy Hematologic Transplant and Endocrine procedures
- 122. Treatment (2) Inoperable or inaccessible tumors may be treated with primary radiation and other systemic therapy:
- 123. Surgical Procedure of Primary Site Brain: Site-specific surgery codes Meninges Brain Spinal cord, cranial nerves, other
- 124. Surgical Procedure of Primary Site C70-0-C70.9, C71.0-C71.9, C72.0-C72.9 (1) Code 10: Tumor destruction, NOS Laser surgery
- 125. Surgical Procedure of Primary Site C70-0-C70.9, C71.0-C71.9, C72.0-C72.9 (2) 20:Local Excision (biopsy) of tumor, lesion, or
- 126. Surgical Procedure of Primary Site C75.1, C75.2, C75.3 (1) Code 10: Local tumor destruction, NOS Code
- 127. Surgical Procedure of Primary Site C75.1, C75.2, C75.3 (2) Code 20: Local tumor excision, NOS Code
- 128. Surgical Procedure of Primary Site C75.1, C75.2, C75.3 (3) Code 25: Laser excision Specimen sent to
- 129. Surgical Procedure of Primary Site C75.1, C75.2, C75.3 (4) Code 40: Total surgical removal of primary
- 130. Surgical Margins of the Primary Site Code final status of surgical margins COC-required data item. Serves
- 131. Scope of Regional Lymph Node Surgery Identifies removal, biopsy, or aspiration of regional lymph node(s): NPCR-,
- 132. Radiation Therapy (1) Radiation codes indicate type of radiation therapy performed as part of the first
- 133. Radiation Therapy (2) Beam radiation Codes 20 – 29: Conventional radiation therapy: from an external beam
- 134. Radiation Therapy (3) Beam radiation Code 32: Conformal radiation Code 40: Particle or proton beam Code
- 135. Radiation Therapy (3) Tumors typically treated with stereotactic radiosurgery include: Acoustic neuroma Chordoma Pineal tumor Astrocytoma
- 136. Radiation Therapy (4) Radioactive implants Code 50: Brachytherapy, radiation implants, radiation seeding, radioactive implants, interstitial implants,
- 137. Radiation Therapy (5) Radioactive implants (continued) Code 52: Intracavitary radiation with high dose rate applicator Code
- 138. Chemotherapy (1) Record type of chemotherapy administered as first course of treatment: Code 01: Chemotherapy, NOS
- 139. Chemotherapy (2) Blood-brain barrier Protects the brain from foreign substances, including chemotherapy. May be disrupted by
- 140. Chemotherapy (3) Interstitial chemotherapy Administered directly to involved tissues. Polymer wafers soaked in a chemotherapeutic agent
- 141. Hormone Therapy Record systemic hormonal agents administered as first course of treatment. Tamoxifen and RU-486 (Mifepristone)
- 142. Immunotherapy (1) Record whether immunotherapeutic agents were administered as first course of treatment: Angiogenesis inhibitors block
- 143. Immunotherapy (2) Gene therapy replaces or repairs the gene responsible for tumor growth. Vaccine therapy allows
- 144. Hematologic Transplant and Endocrine Procedures Identify systemic therapeutic procedures administered as first course of treatment: Code
- 145. Technical Issues Edit Checks NAACCR Edits Committee is developing and modifying data edits to accommodate data
- 146. Technical Issues Data Analysis Recommendations Report and analyze data for non-malignant CNS tumors separately from malignant
- 147. References Manuals, Articles, Reports A Primer of Brain Tumors, 1998; American Brain Tumor Association, Des Plaines,
- 148. References Manuals, Articles, Reports (continued) Fritz A, Percy C, Jack V, Shanmugaratnam K, Sobin V, Parkin
- 149. References Websites American Brain Tumor Association www.abta.org American College of Surgeons, Commission on Cancer Information, Facility
- 150. References Websites (continued) Brain and Neurosurgery Information Center www.brain-surgery.com/index.html Brain and Spinal Cord Tumors: Hope through
- 151. References Websites (continued) College of American Pathologists (CAP), Protocol – Brain ftp://ftp.cap.org/cancerprotocols/Brain03_p.doc Illustrated Glossary of Radiology:
- 152. References Websites (continued) International RadioSurgery Association www.isra.org/index.html National Brain Tumor Radiosurgery Association www.braintumors.com/radiosurgery/radiosrugery.info#TWO NCI Brain Tumor
- 153. References Websites (continued) PDQ Cancer Information Summaries: Adult Treatment www.cancer.gov/cancerinfo/pdq/adulttreatment PDQ Cancer Information Summaries: Pediatric Treatment
- 154. Acknowledgments (1) Prepared by Shannon Vann, CTR for the North American Association of Central Cancer Registries
- 155. Acknowledgments (2) Sponsors Centers for Disease Control and Prevention National Program for Cancer Registries National Cancer
- 156. Acknowledgments (3) CDC National Program of Cancer Registries Planning Committee Kimberly Cantrell Gayle G. Clutter Faye
- 158. Скачать презентацию
































































































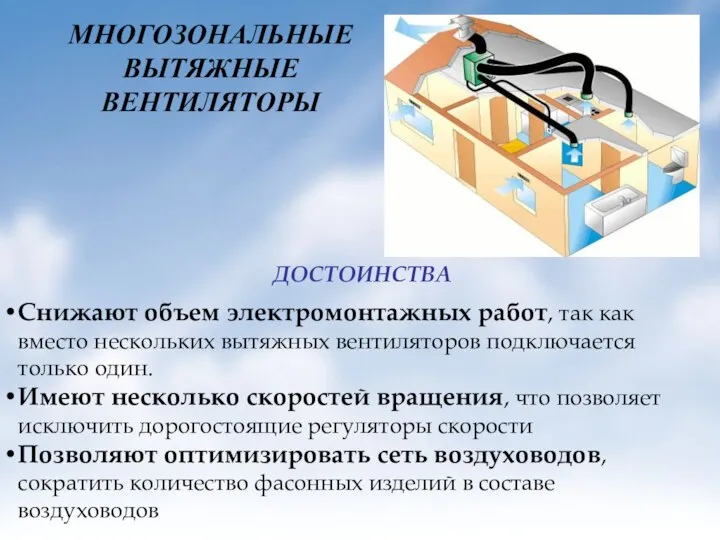 Многозональные вытяжные вентиляторы
Многозональные вытяжные вентиляторы 11 класс
11 класс  Формы государства. Политика
Формы государства. Политика Богослужения суббот Великого поста
Богослужения суббот Великого поста Аполлон и Дафна
Аполлон и Дафна Культура и информатизация
Культура и информатизация  Бетон
Бетон Декоративно – прикладное искусство в жизни человека. Лоскутное шитье. (5 класс) Учитель – технологии Лисичкина Зинаида Алекс
Декоративно – прикладное искусство в жизни человека. Лоскутное шитье. (5 класс) Учитель – технологии Лисичкина Зинаида Алекс Деловая культура Кореи
Деловая культура Кореи (з†бвм-ѓа®ЂЃ¶•≠®•)-КРУГИ ЭЙЛЕРА
(з†бвм-ѓа®ЂЃ¶•≠®•)-КРУГИ ЭЙЛЕРА Презентация на тему "Семинар Методы и формы организации контроля усвоения учащимися учебного материала" - скачать презентаци
Презентация на тему "Семинар Методы и формы организации контроля усвоения учащимися учебного материала" - скачать презентаци лек9структур05прогр
лек9структур05прогр  Международные отношения на современном этапе
Международные отношения на современном этапе Зона комфорта
Зона комфорта  Виктор Михайлович Васнецов
Виктор Михайлович Васнецов Формирование современной городской среды. Город Глазов 2018-2022 годы. Общественная территория «Сквер у музыкальной школы»
Формирование современной городской среды. Город Глазов 2018-2022 годы. Общественная территория «Сквер у музыкальной школы» Теория культур Эдварда Холла
Теория культур Эдварда Холла Parallel programming technologies on hybrid architectures
Parallel programming technologies on hybrid architectures Марченко Мария, 8 «В»
Марченко Мария, 8 «В»  Презентация на тему "Технология проблемного обучения. Метапредметный подход" - скачать презентации по Педагогике
Презентация на тему "Технология проблемного обучения. Метапредметный подход" - скачать презентации по Педагогике Психические познавательные процессы
Психические познавательные процессы Жостовская роспись
Жостовская роспись О маркетинге. О книгах. О принципах. Игорь Манн
О маркетинге. О книгах. О принципах. Игорь Манн Теория и практика решения кейсов по корпоративному праву
Теория и практика решения кейсов по корпоративному праву Политическое сознание
Политическое сознание Государственные и заказы на муниципальном уровне
Государственные и заказы на муниципальном уровне Первый день творения
Первый день творения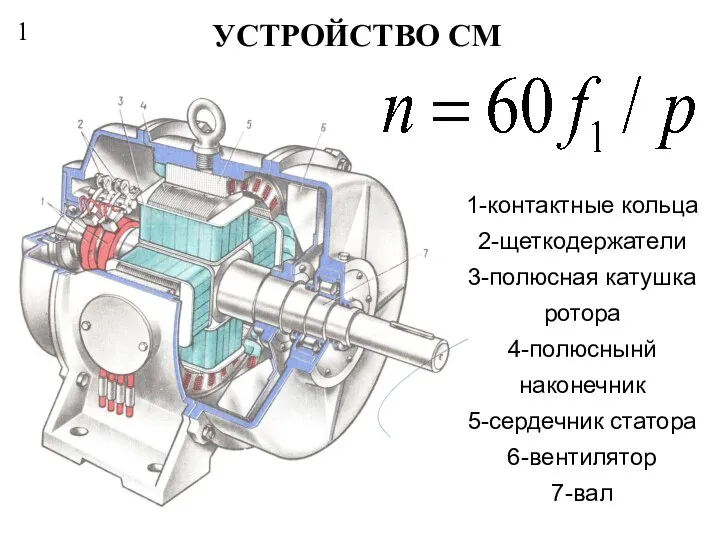 Устройство СМ
Устройство СМ