Содержание
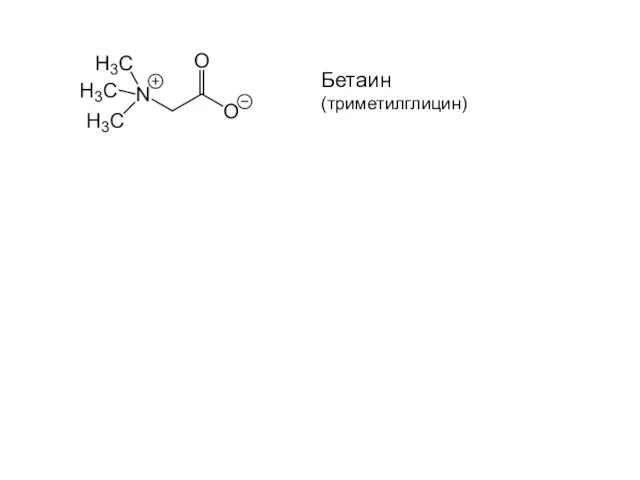
- 3. Бетаин (триметилглицин)
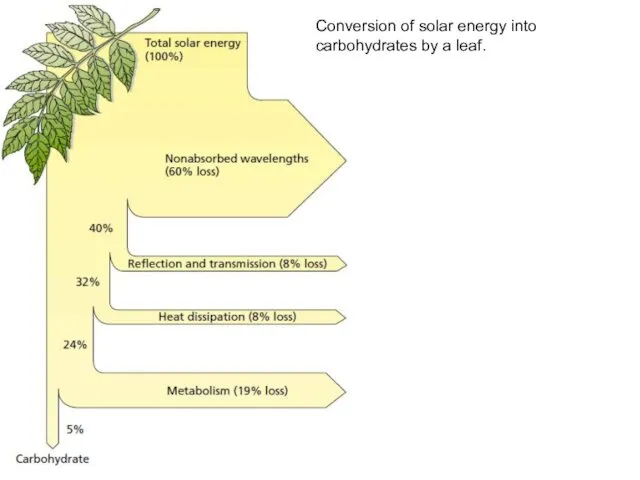
- 4. Conversion of solar energy into carbohydrates by a leaf.
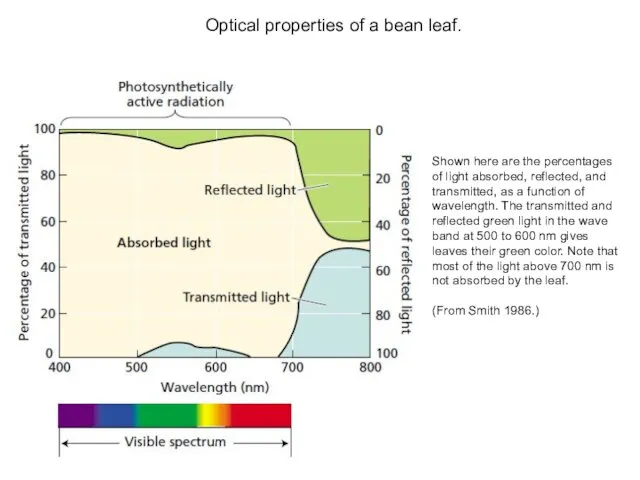
- 5. Shown here are the percentages of light absorbed, reflected, and transmitted, as a function of wavelength.
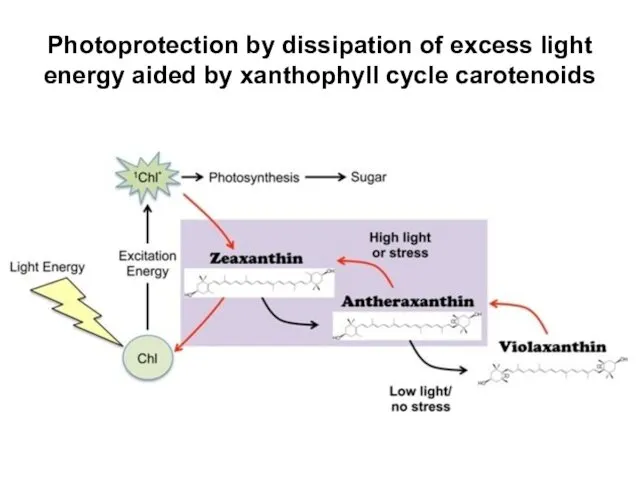
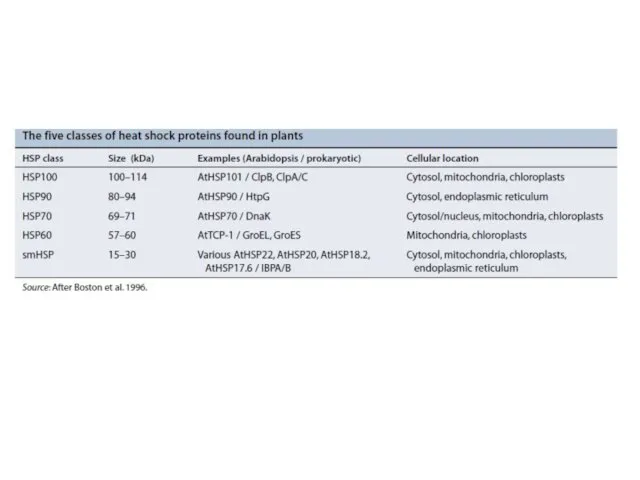
- 6. Photoprotection by dissipation of excess light energy aided by xanthophyll cycle carotenoids
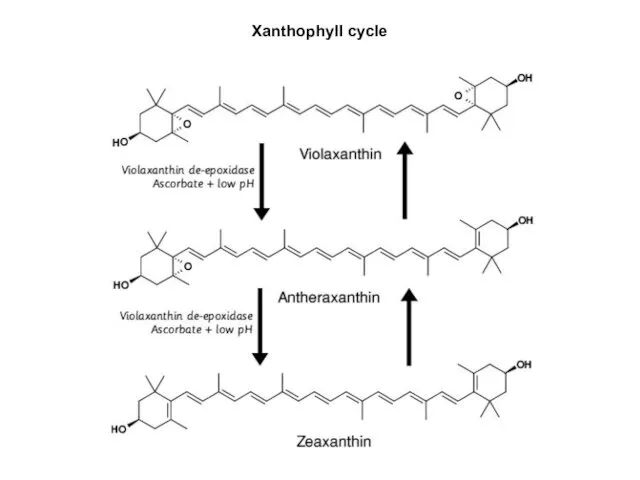
- 7. Xanthophyll cycle
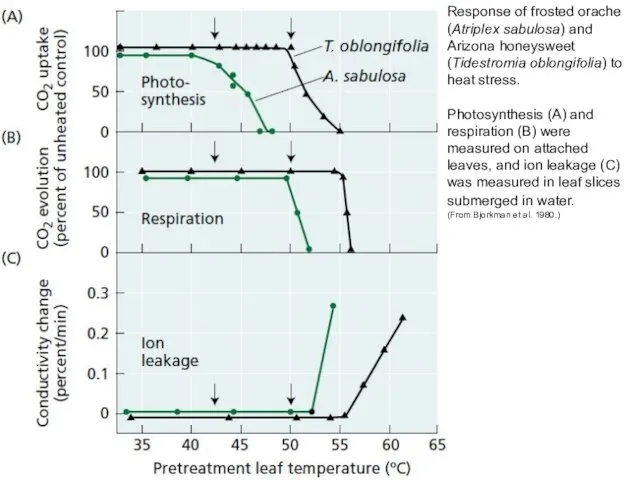
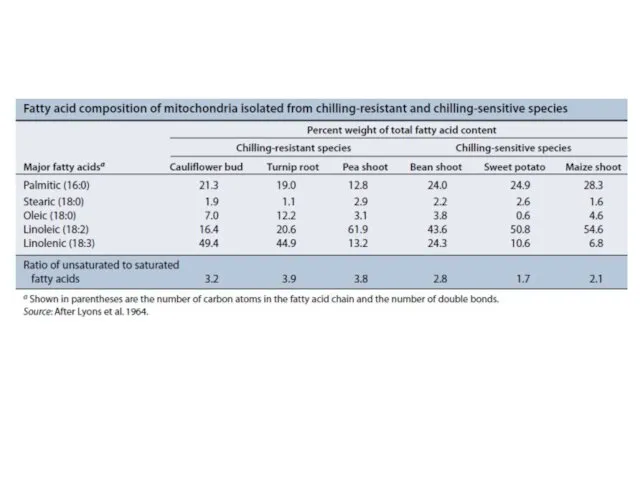
- 9. Response of frosted orache (Atriplex sabulosa) and Arizona honeysweet (Tidestromia oblongifolia) to heat stress. Photosynthesis (A)
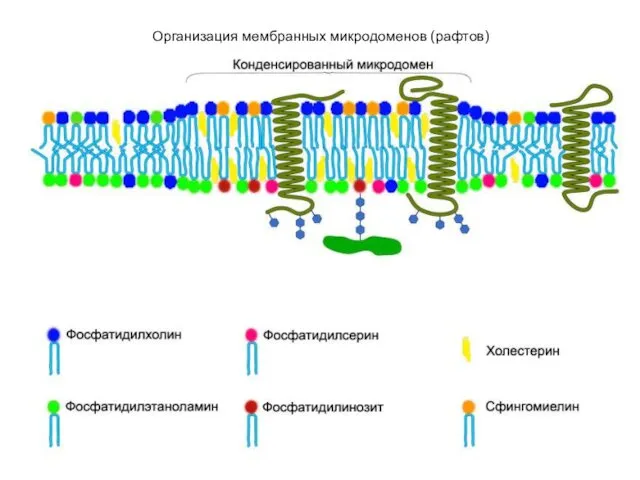
- 10. Организация мембранных микродоменов (рафтов)
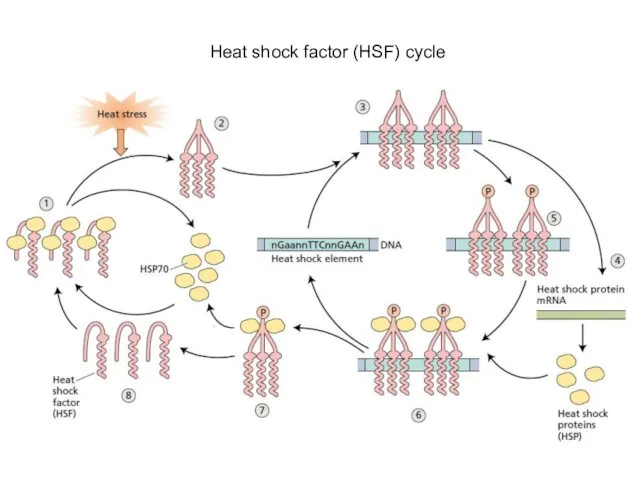
- 13. Heat shock factor (HSF) cycle
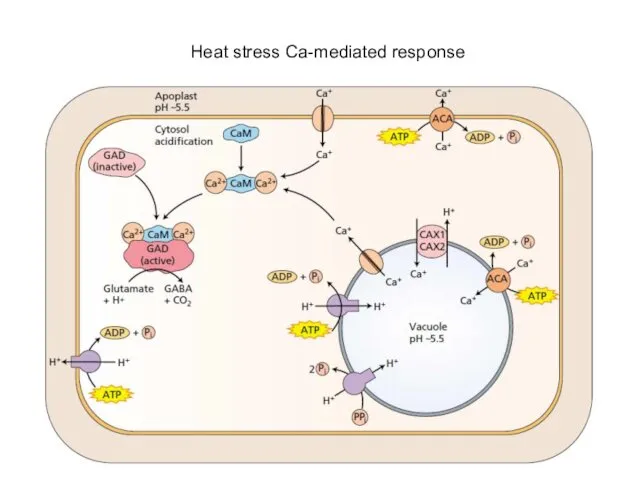
- 14. Heat stress Ca-mediated response
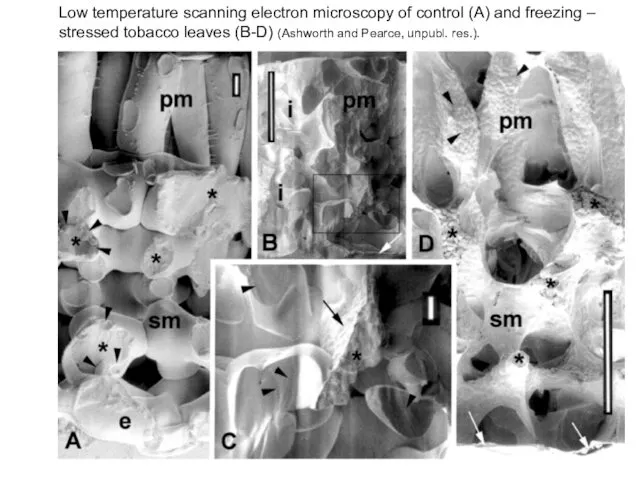
- 15. Low temperature scanning electron microscopy of contro (A) and freezing – stressed tobacco leaves (B-D) (Ashworth
- 16. Low temperature scanning electron microscopy of control (A) and freezing – stressed tobacco leaves (B-D) (Ashworth
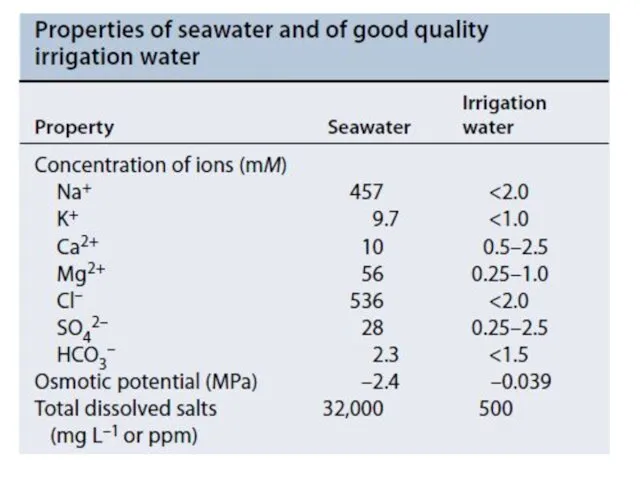
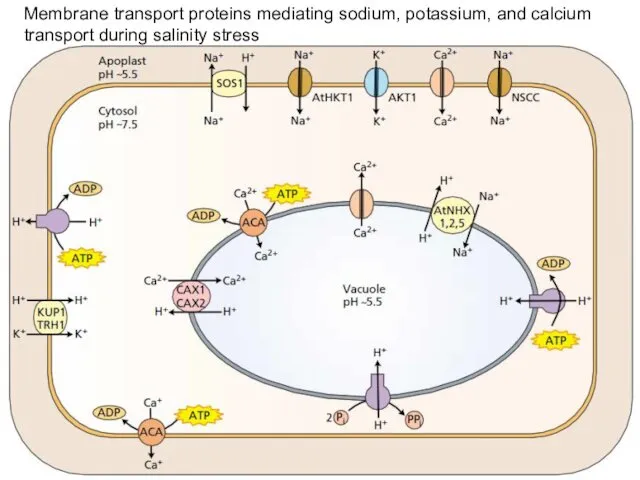
- 19. Membrane transport proteins mediating sodium, potassium, and calcium transport during salinity stress
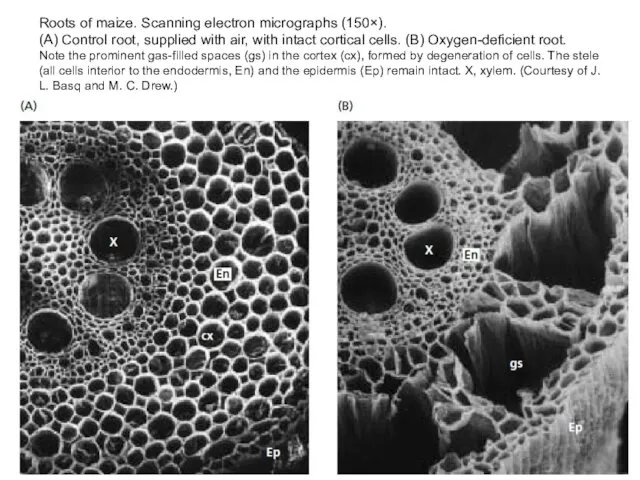
- 20. Roots of maize. Scanning electron micrographs (150×). (A) Control root, supplied with air, with intact cortical
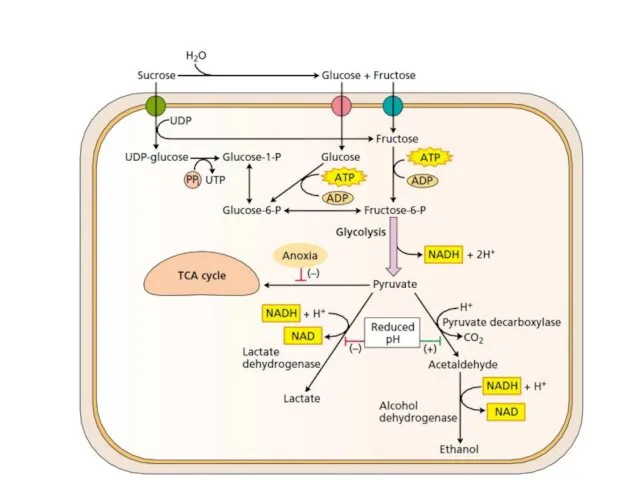
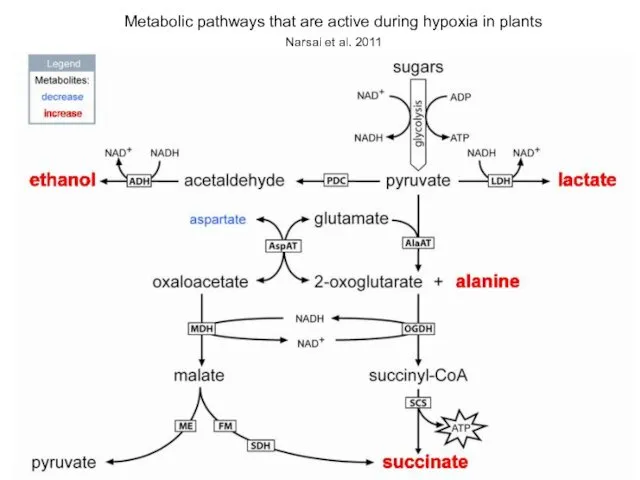
- 22. Metabolic pathways that are active during hypoxia in plants Narsai et al. 2011
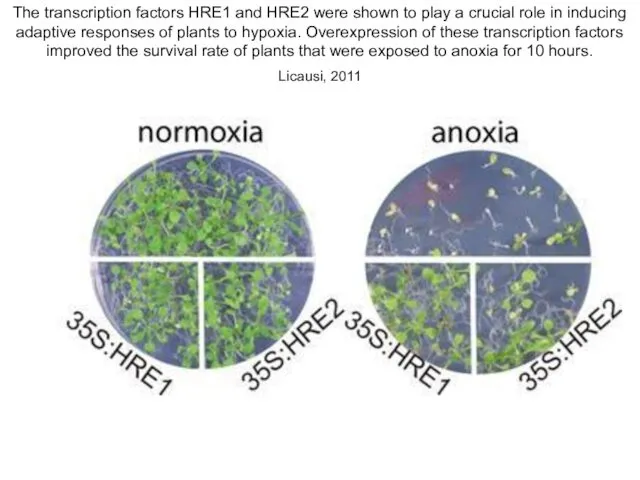
- 23. The transcription factors HRE1 and HRE2 were shown to play a crucial role in inducing adaptive
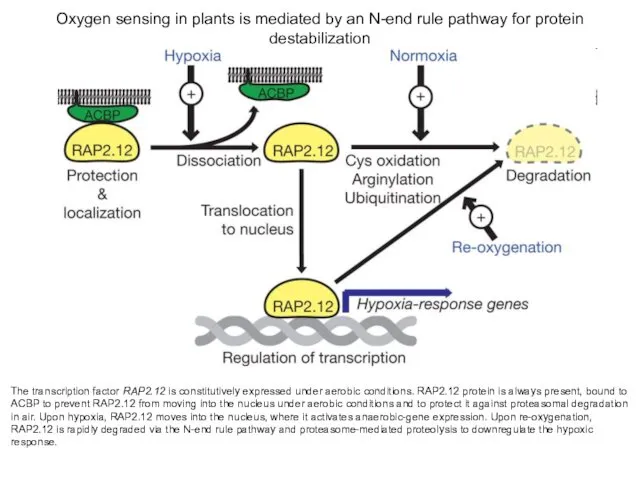
- 24. Oxygen sensing in plants is mediated by an N-end rule pathway for protein destabilization The transcription
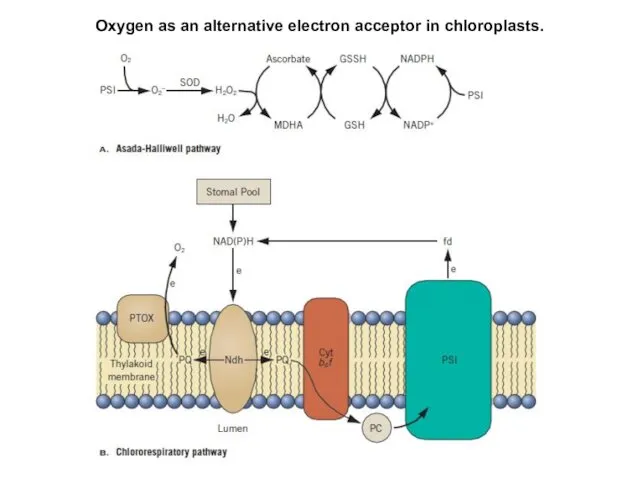
- 25. Oxygen as an alternative electron acceptor in chloroplasts.
- 28. Скачать презентацию

 Курсовая работа. Влияние микроэлементов на физиологические процессы
Курсовая работа. Влияние микроэлементов на физиологические процессы Аскорбиновая кислота. Глютаминовая кислота. Кислота аминокапроновая
Аскорбиновая кислота. Глютаминовая кислота. Кислота аминокапроновая История открытия стекла
История открытия стекла Углерод. Оксиды углерода. Угольная кислота. Карбонаты. Жёсткость воды
Углерод. Оксиды углерода. Угольная кислота. Карбонаты. Жёсткость воды Изомерия алкенов
Изомерия алкенов Модель Андерсона
Модель Андерсона Проблема химического элемента. Концепции структуры химических соединений
Проблема химического элемента. Концепции структуры химических соединений Алюминий (лат. Aluminium)
Алюминий (лат. Aluminium) Методы исследования комплексообразования. Леция 2
Методы исследования комплексообразования. Леция 2 Высокомолекулярные соединения и супрамолекулярные структуры. (Лекция 3)
Высокомолекулярные соединения и супрамолекулярные структуры. (Лекция 3) Александрит
Александрит Изомеризация легких парафиновых углеводородов
Изомеризация легких парафиновых углеводородов Гетероциклические соединения. Синтетические пиретроиды
Гетероциклические соединения. Синтетические пиретроиды Теоретические основы химической технологии
Теоретические основы химической технологии Химия воды
Химия воды Презентация по Химии "Прославившиеся ученые Саратовской области" - скачать смотреть
Презентация по Химии "Прославившиеся ученые Саратовской области" - скачать смотреть  Презентация по Химии "Эффективность снижения общей жесткости воды различными умягчителями, поступающими в продажу" - скачать
Презентация по Химии "Эффективность снижения общей жесткости воды различными умягчителями, поступающими в продажу" - скачать Содержание ртути и её соединений в воздухе при использовании осветительных ламп
Содержание ртути и её соединений в воздухе при использовании осветительных ламп Аттестационная работа. Образовательная программа, элективный курс «Химия в задачах и упражнениях»
Аттестационная работа. Образовательная программа, элективный курс «Химия в задачах и упражнениях» Химиялық рекативтер.стандартты және нормальдық ертінділер дайындау тәртібі мен технологиясы
Химиялық рекативтер.стандартты және нормальдық ертінділер дайындау тәртібі мен технологиясы Миметик глицина на основе производного Гераниола
Миметик глицина на основе производного Гераниола Презентация по Химии "Роль металлов в искусстве" - скачать смотреть
Презентация по Химии "Роль металлов в искусстве" - скачать смотреть  Алюминий в природе
Алюминий в природе Общая характеристика металлов
Общая характеристика металлов Биохимия. Лекция 7. Углеводы
Биохимия. Лекция 7. Углеводы Тағам қоспалары
Тағам қоспалары Биологически важные поли- и гетерофункциональные соединения
Биологически важные поли- и гетерофункциональные соединения Решение 33 задания
Решение 33 задания