Содержание
- 2. Reelin modulates NMDA (N-methyl-D-aspartate) receptor (NMDAR) activity through SRC family tyrosine kinases (SFKs), which is a
- 4. Lipid-associated apolipoprotein E (APOE) could impede reelin-induced signalling by competing for lipoprotein receptor binding3, 3, 88
- 6. a | Reelin binds to lipoprotein receptors, the VLDLR and the APOER2, with high affinity at
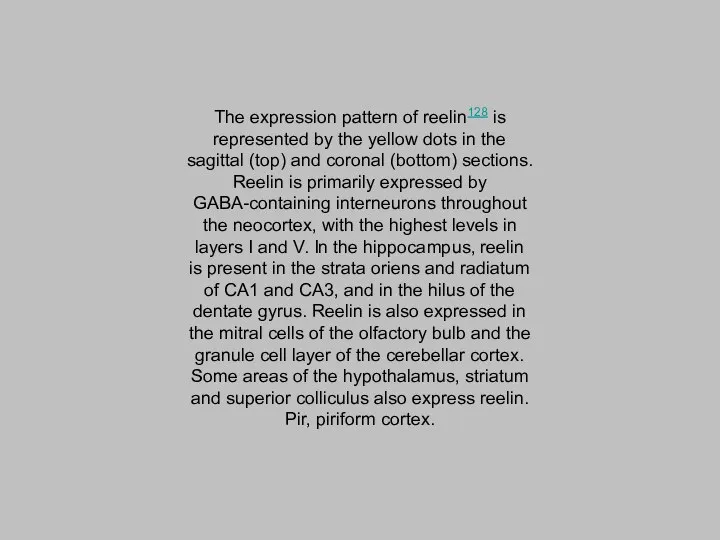
- 8. The expression pattern of reelin128 is represented by the yellow dots in the sagittal (top) and
- 11. Скачать презентацию
Слайд 2
Reelin modulates NMDA (N-methyl-D-aspartate) receptor (NMDAR) activity through SRC family tyrosine
Reelin modulates NMDA (N-methyl-D-aspartate) receptor (NMDAR) activity through SRC family tyrosine
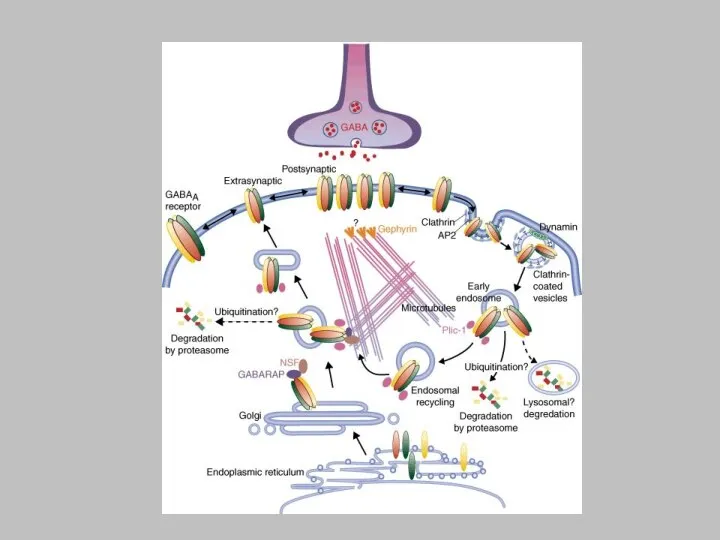
kinases (SFKs), which is a general mechanism utilized by several signalling pathways, including Eph-receptors (Box 1). G-protein-coupled receptors (GPCRs) and metabotropic glutamate receptor 5 (mGluR5) both signal through protein kinase C (PKC) to activate SFKs. Tyrosine phosphorylation of the NMDAR modulates receptor gating properties. PKC129 and D1-type dopamine receptor (D1R)130-mediated signals have been shown to regulate the trafficking of NMDA receptors. APOER2, apolipoprotein E receptor 2; CAK, cell adhesion kinase-; VLDLR, very-low-density lipoprotein receptor.
Слайд 3
Слайд 4
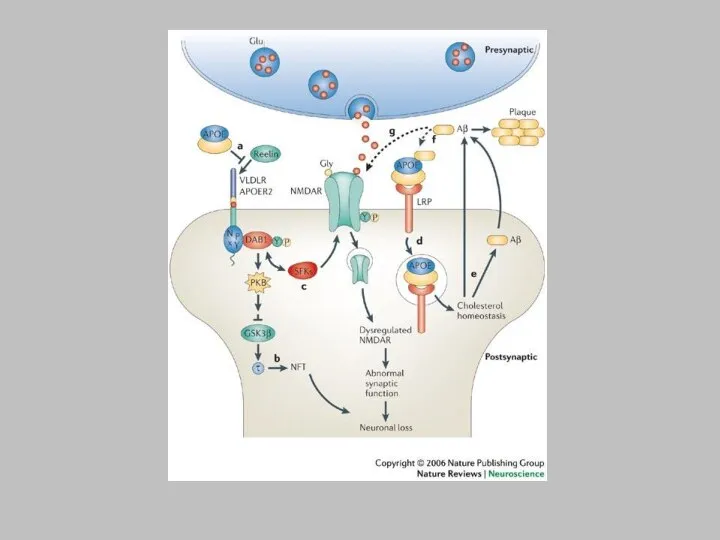
Lipid-associated apolipoprotein E (APOE) could impede reelin-induced signalling by competing for
Lipid-associated apolipoprotein E (APOE) could impede reelin-induced signalling by competing for
lipoprotein receptor binding3, 3, 88 (a). Impaired reelin signalling results in elevated phosphorylation4, which could in turn lead to the formation of neurofibrillary tangles (NFTs) (b). APOE might also inhibit reelin-mediated potentiation of NMDA (N-methyl-D-aspartate) receptor (NMDAR) activity and synaptic plasticity (c). After binding to low-density lipoprotein receptor (LDLR)-related proteins (LRP), APOE-containing lipoproteins undergo endocytosis (d). Different APOE isoforms might differentially affect intracellular trafficking131, 131, 132 of the NMDAR (through association with APOE receptors84, 84, 87) and thereby affect its functional availability. Cholesterol homeostasis has a profound impact on the production and trafficking of the amyloid- (A) peptide6, 6, 51, 6, 51, 133 (e). Extracellular A could associate with APOE and get cleared through receptor-mediated endocytosis (f). Extracellular A represses NMDAR activity52 directly, but also by promoting the endocytosis of NMDARs120 (g). Reduced glutamatergic transmission could result in impaired synaptic plasticity and promote neuronal loss and dementia. APOER2, APOE receptor 2; DAB1, disabled 1; GSK3b;, gylcogen synthase kinase 3; PKB, protein kinase B; SFKs, SRC family tyrosine kinases; VLDLR, very-low-density lipoprotein receptor.
Слайд 5
Слайд 6
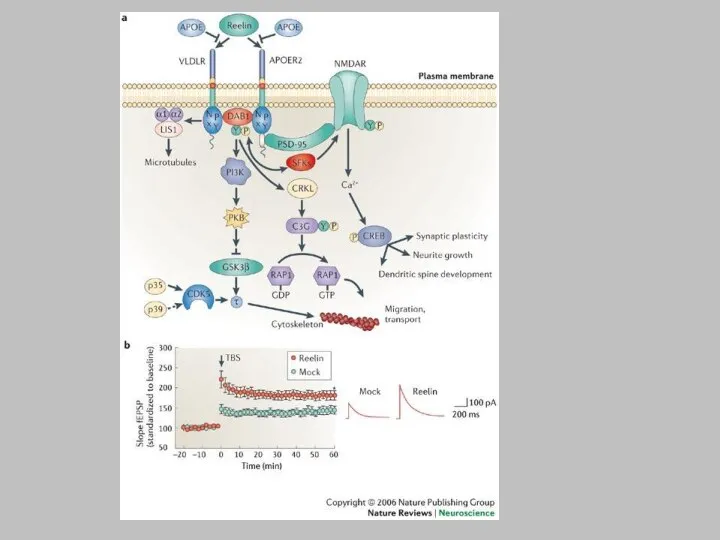
a | Reelin binds to lipoprotein receptors, the VLDLR and the
a | Reelin binds to lipoprotein receptors, the VLDLR and the
APOER2, with high affinity at the cell surface. Binding of reelin to the receptors induces feed-forward activation of DAB1, an adaptor protein that interacts with NPxY motifs in both receptor tails. The clustering of DAB1 activates SRC family tyrosine kinases (SFKs), which potentiates tyrosine phosphorylation of DAB1 (Refs 32a | Reelin binds to lipoprotein receptors, the VLDLR and the APOER2, with high affinity at the cell surface. Binding of reelin to the receptors induces feed-forward activation of DAB1, an adaptor protein that interacts with NPxY motifs in both receptor tails. The clustering of DAB1 activates SRC family tyrosine kinases (SFKs), which potentiates tyrosine phosphorylation of DAB1 (Refs 32, 33). Phosphorylated DAB1 further activates phosphatidylinositol-3-kinase (PI3K) and subsequently protein kinase B (PKB)121. PKB activation inhibits the activity of glycogen synthase kinase 3 (GSK3). As a result, phosphorylation of is reduced, promoting microtubule stability. Tyrosine-phosphorylated (YP) DAB1 also recruits CRK-like (CRKL), which induces phosphorylation of a guanine nucleotide exchange factor, C3G122. Activated C3G promotes the formation of RAP1-GTP, which controls actin cytoskeleton rearrangement. Lissencephaly 1 (LIS1) is another binding partner of tyrosine-phosphorylated DAB1. It associates with a-subunits to form a Pafah1b complex, which regulates microtubule dynamics35, 35, 123, 35, 123, 124, 35, 123, 124, 125, 35, 123, 124, 125, 126, 35, 123, 124, 125, 126, 127. Cyclin-dependent kinase 5 (CDK5) acts in parallel with reelin on numerous substrates, including microtubules. p35 and p39 are activating subunits of CDK5. APOER2 associates with postsynaptic density protein 95 (PSD-95), an abundant scaffolding protein in the PSD, through an alternatively spliced exon. This interaction is crucial for the coupling of the reelin signalling complex to the NMDA (N-methyl-D-aspartate) receptor (NMDAR)84, 84, 86. Reelin-activated SFKs tyrosine phosphorylate the NMDAR on NR2 subunits, resulting in the potentiation of NMDAR-mediated Ca2+ influx. Elevated intracellular Ca2+ can activate the transcription factor cyclic AMP-response element binding protein (CREB), thereby potentially initiating the expression of genes that are important for synaptic plasticity, neurite growth and dendritic spine development86. b | Reelin treatment potentiates long-term potentiation in wild-type hippocampal slices82. Reelin treatment also enhances NMDAR-mediated whole cell current in wild-type CA1 pyramidal neurons86. Traces were recorded at a holding potential of +40 mV. fESP, field excitatory postsynaptic potential; TBS, theta burst stimulation. Panel a modified, with permission from Ref. 84. Traces were recorded at a holding potential of +40 mV. fESP, field excitatory postsynaptic potential; TBS, theta burst stimulation. Panel a modified, with permission from Ref. 84 © (2005) Elsevier Science. Panels a and b (right) modified, with permission, from Ref. 82. Traces were recorded at a holding potential of +40 mV. fESP, field excitatory postsynaptic potential; TBS, theta burst stimulation. Panel a modified, with permission from Ref. 84 © (2005) Elsevier Science. Panels a and b (right) modified, with permission, from Ref. 82 © (2002) American Society for Biochemistry and Molecular Biology. Panel b (left) modified from Ref. 86 © (2005) Society for Neuroscience.
Слайд 7
Слайд 8
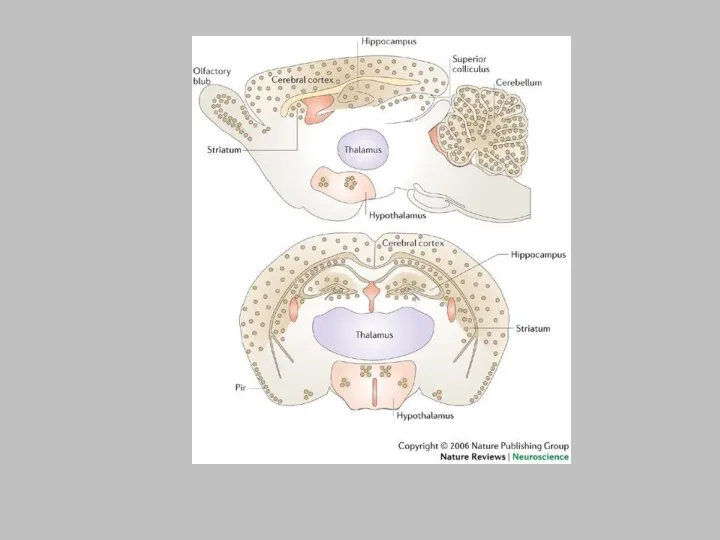
The expression pattern of reelin128 is represented by the yellow dots
The expression pattern of reelin128 is represented by the yellow dots
in the sagittal (top) and coronal (bottom) sections. Reelin is primarily expressed by GABA-containing interneurons throughout the neocortex, with the highest levels in layers I and V. In the hippocampus, reelin is present in the strata oriens and radiatum of CA1 and CA3, and in the hilus of the dentate gyrus. Reelin is also expressed in the mitral cells of the olfactory bulb and the granule cell layer of the cerebellar cortex. Some areas of the hypothalamus, striatum and superior colliculus also express reelin. Pir, piriform cortex.
Слайд 9
- Предыдущая
Neurons Stephen Richards 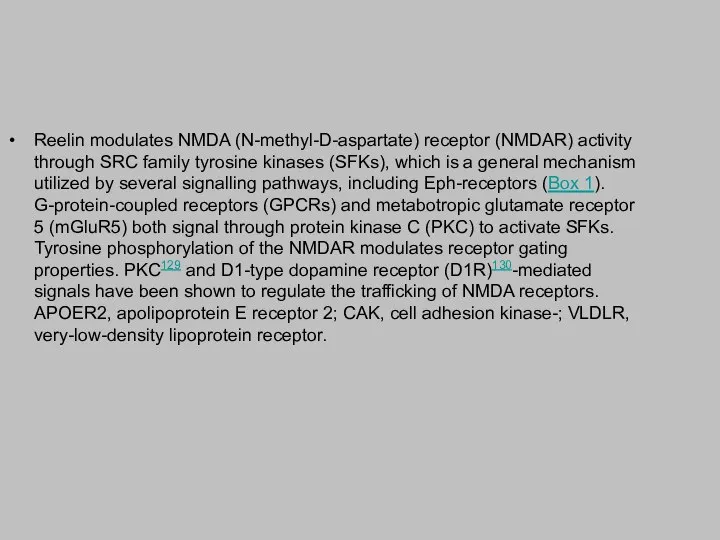


 Короткие замыкания в системах электроснабжения. (Лекция 4)
Короткие замыкания в системах электроснабжения. (Лекция 4) «Что говорят ваши клиенты сегодня?»
«Что говорят ваши клиенты сегодня?» Сети DWDM. (Лекция 9)
Сети DWDM. (Лекция 9) Технико-экономическое обоснование выбора класса напряжения эск 6/10 кв в условиях изолированной энергетики рс(я)
Технико-экономическое обоснование выбора класса напряжения эск 6/10 кв в условиях изолированной энергетики рс(я) Технология проектного обучения как способ формирования компетентности в сфере самостоятельной познавательной деятельности и фа
Технология проектного обучения как способ формирования компетентности в сфере самостоятельной познавательной деятельности и фа Презентация на тему "Болезни органов дыхания 1" - скачать презентации по Медицине
Презентация на тему "Болезни органов дыхания 1" - скачать презентации по Медицине Бизнес Сервис Центр Харьков. Инструкция по выставлению осуществленных рейсов ТЭК на оплату (блок документы)
Бизнес Сервис Центр Харьков. Инструкция по выставлению осуществленных рейсов ТЭК на оплату (блок документы) скрипт
скрипт Презентация "Русская народная вышивка (4 класс)" - скачать презентации по МХК
Презентация "Русская народная вышивка (4 класс)" - скачать презентации по МХК Веб-дизайн
Веб-дизайн Тема урока «Закрепление изученного материала по теме «Безударные гласные» Презентация к уроку русского языка для 2 класса Учите
Тема урока «Закрепление изученного материала по теме «Безударные гласные» Презентация к уроку русского языка для 2 класса Учите Искусственный интеллект. Тест Тьюринга
Искусственный интеллект. Тест Тьюринга Тенденции налогообложения в РФ
Тенденции налогообложения в РФ Косторезная фабрика в Тобольске
Косторезная фабрика в Тобольске Система менеджмента качества
Система менеджмента качества Кто он, современный подросток? Отдел психолого-педагогического Сопровождения Центра «Созвездие» 2010г.
Кто он, современный подросток? Отдел психолого-педагогического Сопровождения Центра «Созвездие» 2010г. Лучшие из лучших
Лучшие из лучших Введение в искусственный интеллект
Введение в искусственный интеллект Написание сочинения-рассуждения на ЕГЭ по русскому языку с помощью шаблона
Написание сочинения-рассуждения на ЕГЭ по русскому языку с помощью шаблона Психолого-педагогическая помощь детям с искажённым развитием (ранний детский аутизм) Выполнила: Черепанова А.С.
Психолого-педагогическая помощь детям с искажённым развитием (ранний детский аутизм) Выполнила: Черепанова А.С. Эффективные стратегии обработки деталей на n станках (n=1, 2, …)
Эффективные стратегии обработки деталей на n станках (n=1, 2, …) Analysis and Design of Data Systems. Relational Algebra 2 (Lecture 18)
Analysis and Design of Data Systems. Relational Algebra 2 (Lecture 18) Презентация Научное исследование как форма существования и развития науки
Презентация Научное исследование как форма существования и развития науки  Метод Мерцалова
Метод Мерцалова БЕЗОПАСНОСТЬ ЖИЗНЕДЕЯТЕЛЬНОСТИ НА ПРЕДПРИЯТИЯХ СВЯЗИ
БЕЗОПАСНОСТЬ ЖИЗНЕДЕЯТЕЛЬНОСТИ НА ПРЕДПРИЯТИЯХ СВЯЗИ Презентация на тему: Фольклор
Презентация на тему: Фольклор Деятельность Росстата
Деятельность Росстата День защитника Отечества Интерактивный классный час Учитель ГОУ школа № 688 Санкт-Петербург Гаврилова Ольга Геннадьевна
День защитника Отечества Интерактивный классный час Учитель ГОУ школа № 688 Санкт-Петербург Гаврилова Ольга Геннадьевна