Содержание
- 2. Disperse system is a system consisting of two or more phases. One of them which mass
- 3. Classification of disperse systems 1. According to a disperse phase dispersion degree : coarsely dispersed (particle
- 4. Classification of disperse systems 2. According to the aggregate state of the disperse phase and the
- 5. Methods of colloidal solutions preparation Dispergation methods (breaking up larger particles to the colloid dispersion degree)
- 6. Colloidal solutions purification methods Dialysis Electrodialysis Ultrafiltration
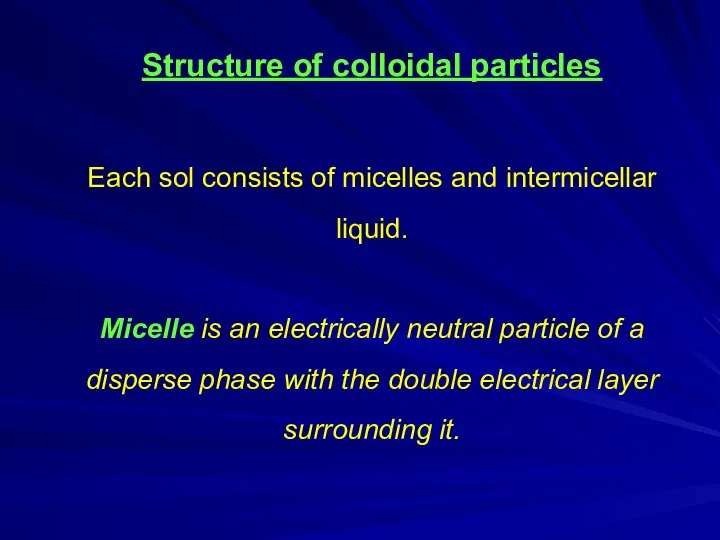
- 7. Structure of colloidal particles Each sol consists of micelles and intermicellar liquid. Micelle is an electrically
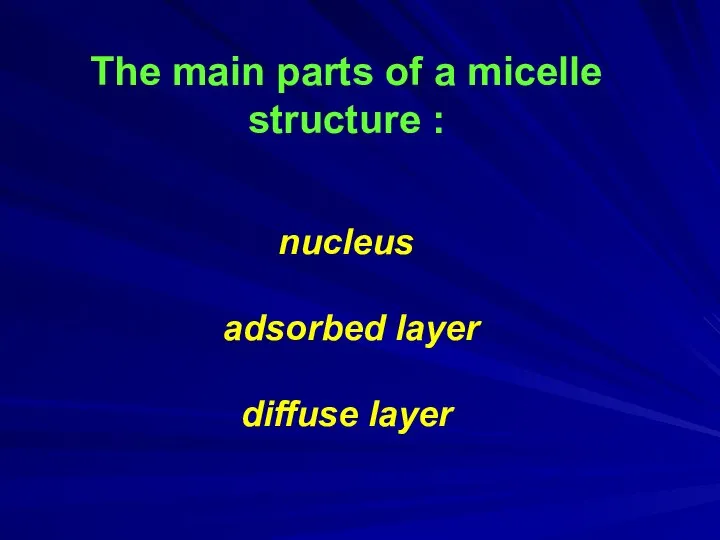
- 8. The main parts of a micelle structure : nucleus adsorbed layer diffuse layer
- 9. The process of settling of disperse phase particles under the action of forces of different nature
- 10. The process of colloidal particles combining into larger aggregates due to loss of aggregation stability is
- 11. Coarsely dispersed systems Aerosols are disperse systems with a gas disperse medium and a liquid or
- 12. Coarsely dispersed systems Pastes are coarsely dispersed systems with high concentration of a solid disperse phase
- 14. Скачать презентацию









 Строение соединений d-элементов
Строение соединений d-элементов Aлканы. Определение. Общая формула класса углеводородов
Aлканы. Определение. Общая формула класса углеводородов Углерод. Аллотропные модификации
Углерод. Аллотропные модификации Ендотермічні реакції на службі людини
Ендотермічні реакції на службі людини Фазовая диаграмма GaAs. Ретроградная растворимость. Селективная летучесть мышьяка из расплава. Методы борьбы с этими проблемами
Фазовая диаграмма GaAs. Ретроградная растворимость. Селективная летучесть мышьяка из расплава. Методы борьбы с этими проблемами Исследовательская работа «Волшебная соль»
Исследовательская работа «Волшебная соль» Фенол. Получение и использование, физические и химические свойства. Биологическая роль
Фенол. Получение и использование, физические и химические свойства. Биологическая роль Уран – периодты жүйедегі атомдық номері 92 болатын химиялық элемент
Уран – периодты жүйедегі атомдық номері 92 болатын химиялық элемент Презентация по Химии "Высшие природные полимеры - Белки и Нуклеиновые кислоты" - скачать смотреть
Презентация по Химии "Высшие природные полимеры - Белки и Нуклеиновые кислоты" - скачать смотреть  Введение. Развитие химической технологии как науки
Введение. Развитие химической технологии как науки Антибиотики-аминогликозиды: получение, свойства, исследование и применение. Связь между химическим строением и действием
Антибиотики-аминогликозиды: получение, свойства, исследование и применение. Связь между химическим строением и действием Вода - растворитель
Вода - растворитель Беседа с в 11 классе
Беседа с в 11 классе Простые и сложные вещества. Металлы и неметаллы. Бинарные соединения
Простые и сложные вещества. Металлы и неметаллы. Бинарные соединения Галогены. Основные химические свойства. Качественные реакции
Галогены. Основные химические свойства. Качественные реакции Алкалоидтар. Алкалоидтар туралы жалпы түсінік
Алкалоидтар. Алкалоидтар туралы жалпы түсінік Актиний
Актиний Краткий очерк истории развития химии
Краткий очерк истории развития химии Комплексные соединения хлоридов европия и гадолиния с салициловой кислотой
Комплексные соединения хлоридов европия и гадолиния с салициловой кислотой Неметаллы
Неметаллы Конфигурация макромолекулы
Конфигурация макромолекулы Группа веществ, требующих особых методов изолирования. Характеристика соединений. Токсикологическое значение
Группа веществ, требующих особых методов изолирования. Характеристика соединений. Токсикологическое значение Содержание витамина С (аскорбиновой кислоты) в натуральных и консервированных соках.
Содержание витамина С (аскорбиновой кислоты) в натуральных и консервированных соках. Азотсодержащие вещества. Амины
Азотсодержащие вещества. Амины Открытая школа по химии
Открытая школа по химии Предмет и задачи биохимии. Белки. (Лекция 1)
Предмет и задачи биохимии. Белки. (Лекция 1) Radical Approach to the Chiral Quaternary Center in Asperaculin A: Synthesis of 9‑Deoxyasperaculin A
Radical Approach to the Chiral Quaternary Center in Asperaculin A: Synthesis of 9‑Deoxyasperaculin A Презентация по Химии "Патофизиология аритмий сердца ПАТОФИЗИОЛОГИЯ АРИТМИЙ СЕРДЦА" - скачать смотреть бесплатно
Презентация по Химии "Патофизиология аритмий сердца ПАТОФИЗИОЛОГИЯ АРИТМИЙ СЕРДЦА" - скачать смотреть бесплатно