Содержание
- 2. Agenda Functions of Proteins Overview of Protein Structure - levels of organization of protein molecules Primary
- 3. 1. Functions of Proteins Proteins perform the following functions: 1. Structural: in connective tissue - collagen,
- 4. 3. Signaling (Hormonal) function (Peptide hormones or protein hormones): Regulation and coordination of metabolism in different
- 5. 8. Regulatory Proteins - regulate genes expression; 9. Proteins-toxins: pseudomonas exotoxin (PE), diphtheria toxin (DT), etc.
- 6. Resilin provides soft rubber-elasticity to mechanically active organs and tissue. It helps insects to flap the
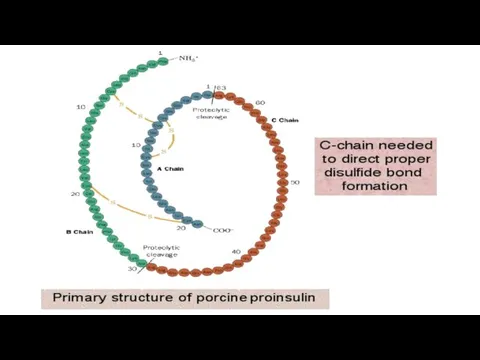
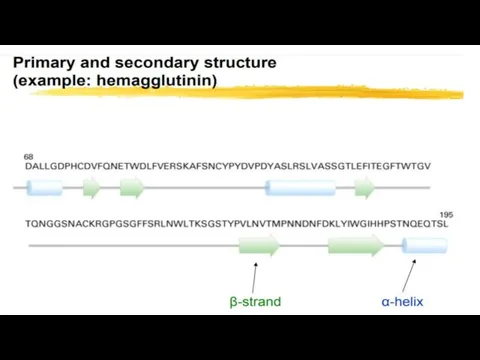
- 8. 2. STRUCTURE – LEVELS OF PROTEIN Molecule ORGANIZATION PRIMARY STRUCTURE OF PROTEINS - This is the
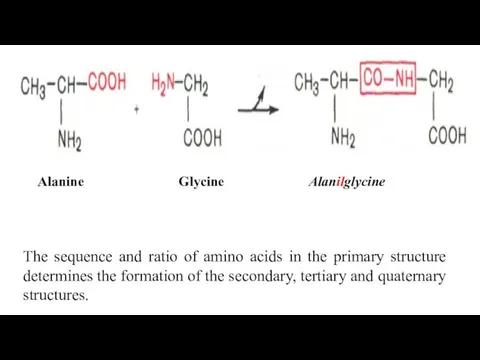
- 9. Alanine Glycine Alanilglycine The sequence and ratio of amino acids in the primary structure determines the
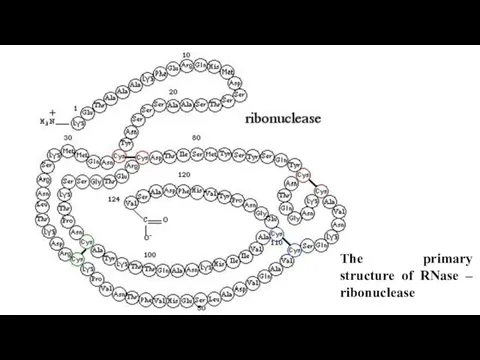
- 11. The primary structure of RNase – ribonuclease
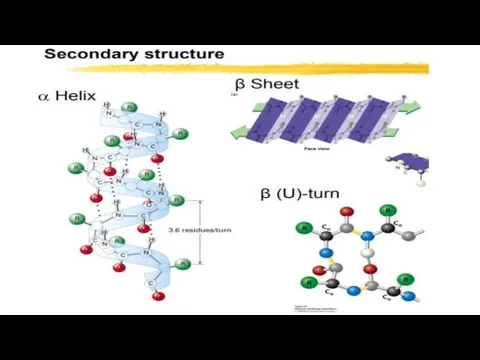
- 12. SECONDARY STRUCTURE: Polypeptide chains can Fold into Regular structures. By this structure of a protein a
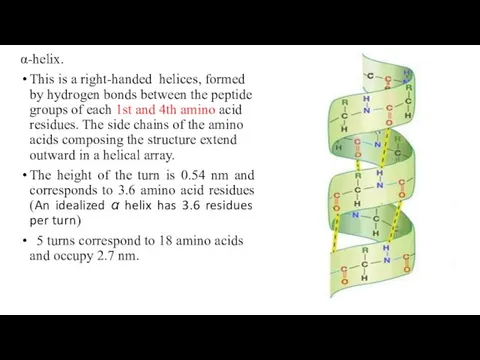
- 14. α-helix. This is a right-handed helices, formed by hydrogen bonds between the peptide groups of each
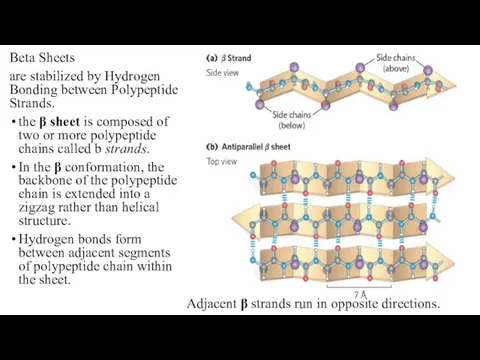
- 16. Beta Sheets are stabilized by Hydrogen Bonding between Polypeptide Strands. the β sheet is composed of
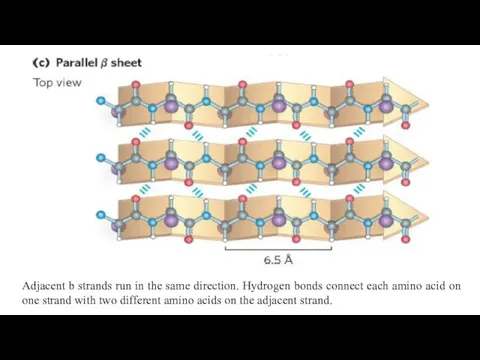
- 17. Adjacent b strands run in the same direction. Hydrogen bonds connect each amino acid on one
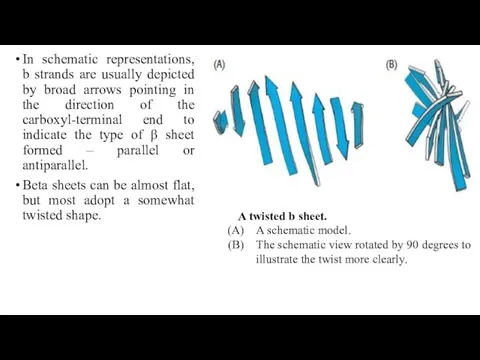
- 18. In schematic representations, b strands are usually depicted by broad arrows pointing in the direction of
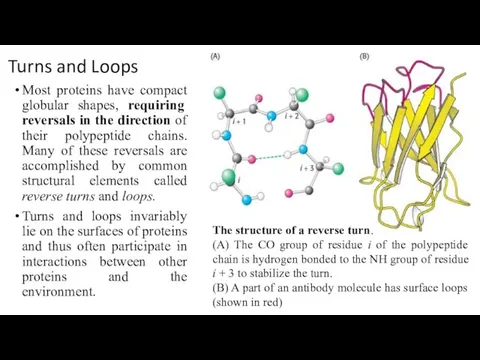
- 19. Turns and Loops Most proteins have compact globular shapes, requiring reversals in the direction of their
- 20. Tertiary Structure: water-soluble Proteins fold into Compact structures The tertiary structure, refers to the spatial arrangement
- 21. Some polypeptide chains fold into two or more compact regions that may be connected by a
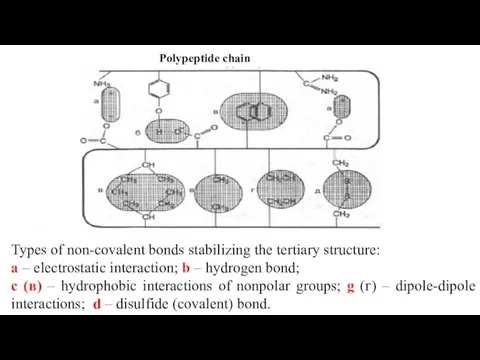
- 22. Bonds involved in the formation of the tertiary structure Various bonds are involved in the formation
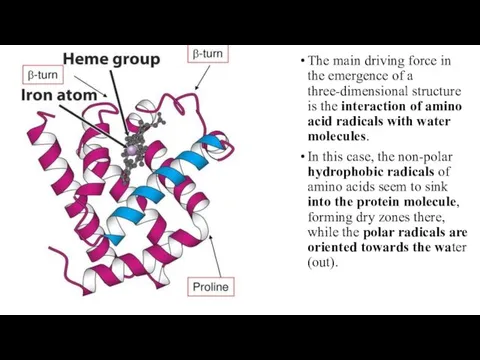
- 23. The main driving force in the emergence of a three-dimensional structure is the interaction of amino
- 24. Polypeptide chain Types of non-covalent bonds stabilizing the tertiary structure: a – electrostatic interaction; b –
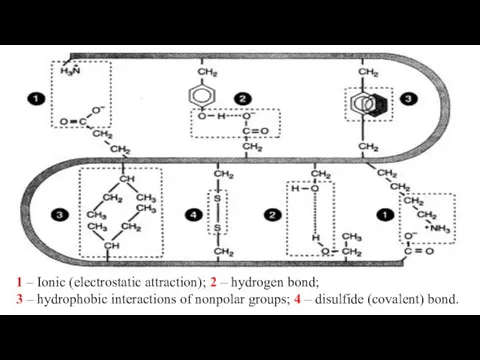
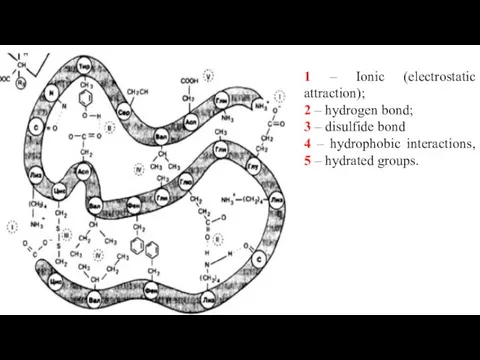
- 25. 1 – Ionic (electrostatic attraction); 2 – hydrogen bond; 3 – hydrophobic interactions of nonpolar groups;
- 26. 1 – Ionic (electrostatic attraction); 2 – hydrogen bond; 3 – disulfide bond 4 – hydrophobic
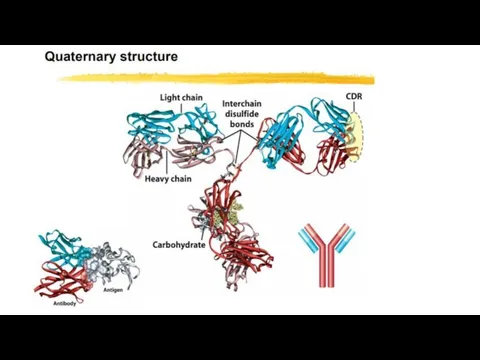
- 27. Quaternary Structure: Multiple Polypeptide Chains Can Assemble into a Single Protein Proteins consisting of more than
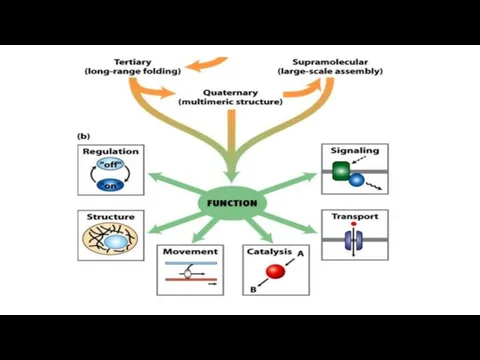
- 29. SUMMARY on Protein Tertiary and Quaternary Structures Tertiary structure is the complete three-dimensional structure of a
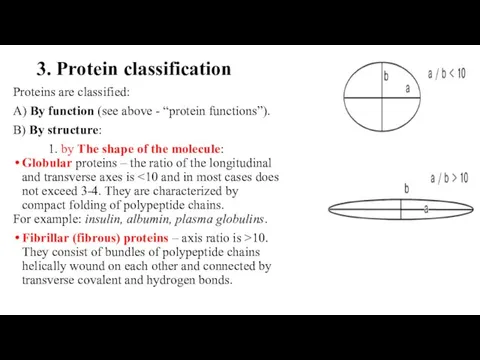
- 30. 3. Protein classification Proteins are classified: A) By function (see above - “protein functions”). B) By
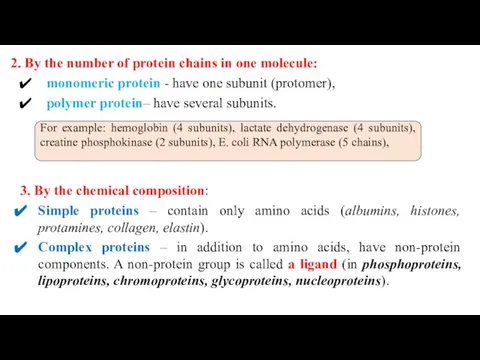
- 31. 2. By the number of protein chains in one molecule: monomeric protein - have one subunit
- 33. Скачать презентацию










 Природные и попутные нефтяные газы
Природные и попутные нефтяные газы Майлардың анықтамасы
Майлардың анықтамасы Общая радиохимия. Свойства радиоколлоидов
Общая радиохимия. Свойства радиоколлоидов Строение атома углерода
Строение атома углерода Да здравствует мыло душистое
Да здравствует мыло душистое Химические и физико-химические методы анализа. Сущность и методы качественного анализа
Химические и физико-химические методы анализа. Сущность и методы качественного анализа Фосфорні добрива. Технології збагачення фосфоровмісної сировини
Фосфорні добрива. Технології збагачення фосфоровмісної сировини Уравнения химических реакций
Уравнения химических реакций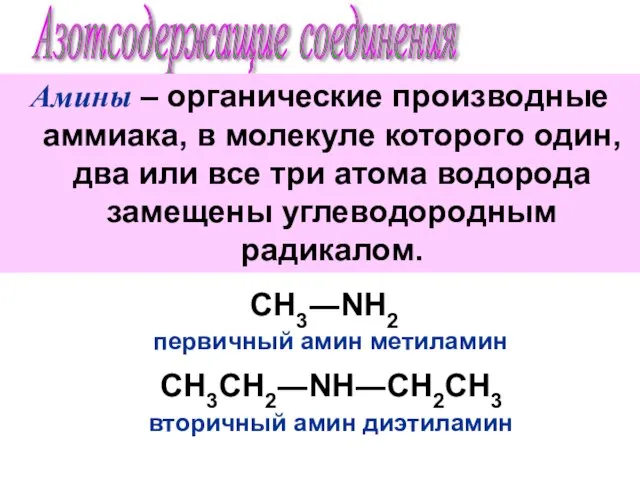 Азотсодержащие соединения. Амины
Азотсодержащие соединения. Амины Оборудование для дистилляции мисцеллы
Оборудование для дистилляции мисцеллы Гидролиз. Сущность процесса гидролиза
Гидролиз. Сущность процесса гидролиза Электронные эффекты заместителей. Типы химических реакций
Электронные эффекты заместителей. Типы химических реакций Исследовательский проект «Кристаллы»
Исследовательский проект «Кристаллы» Минералы. Классы минералов
Минералы. Классы минералов Спирты
Спирты Гидролиз. Виды гидролиза
Гидролиз. Виды гидролиза Пищевые добавки
Пищевые добавки Основания и кислоты. Тема 2
Основания и кислоты. Тема 2 Зміна ліпідів за технологічної обробки
Зміна ліпідів за технологічної обробки Презентация по Химии "Степень окисления" - скачать смотреть бесплатно_
Презентация по Химии "Степень окисления" - скачать смотреть бесплатно_ Презентация по Химии "Высшие природные полимеры - Белки и Нуклеиновые кислоты" - скачать смотреть
Презентация по Химии "Высшие природные полимеры - Белки и Нуклеиновые кислоты" - скачать смотреть  Области применения спиртов
Области применения спиртов Применение синтетических полимеров в вооружении
Применение синтетических полимеров в вооружении Пластик, пластмасса
Пластик, пластмасса Распознавание химических соединений
Распознавание химических соединений Основы безопасности при уничтожении химического оружия
Основы безопасности при уничтожении химического оружия Сера и ее аллотропные модификации. (9 класс)
Сера и ее аллотропные модификации. (9 класс) Виды химической связи
Виды химической связи