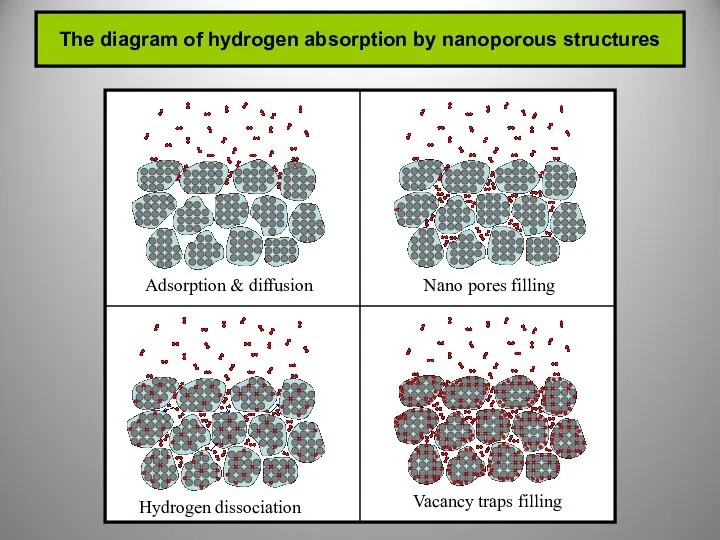
Conventional and nonconventional hydrogen storages.
Storage in high-pressure tanks – up to
700 atmospheres.
Disadvantages – spontaneous leak of hydrogen and high risk of depressurization.
Storage in liquid state– (-252°С).
Disadvantages – thigh cost of equipment for hydrogen storage and cooling, evaporation and high risk of depressurization.
Hydrogen storage in solid state.
Requirements.
Gravimetric capacitance- > 6 weight % H2, Hydrogen pressure at its saturation - < 3 МPа,
Hydrogenation time - < 5 minutes, Temperature of hydrogen desorption - < 85°С
Porous
(physical adsorption)
1. Carbon nanostructures
Nanotubes (single-layer, multilayer),
nanofibers, fullerene, graphene,
activated carbon.
2. Metal - organic structures
MOF-5,177 (Zn4O-[O2C-C6H4-CO2]2),
MIL-53,101(Cr,Al,O [O2C-C6H4-CO2]2),
IMOF-1,3,12 (Zn4O-CxHy(CO2)2)
Compact
(chemical adsorption)
Mg - based hydrides
MgH2 – (Ti, V, Ni, Cu, Fe, Mn),
MgH2 – (V2O5, Nb2O5, Fe2O3, Al2O3, TiO2)
Complex hydrides
NaAlH6, LiAlH4, KAlH4
3. LiN - based hydrides
LiNH2, Li2NH, Li2MgN2H2, Li3BN2H8
4. Intermetallic compounds
LaNi5, FeTi, TiVCr, TiZrNi, TiCrMn
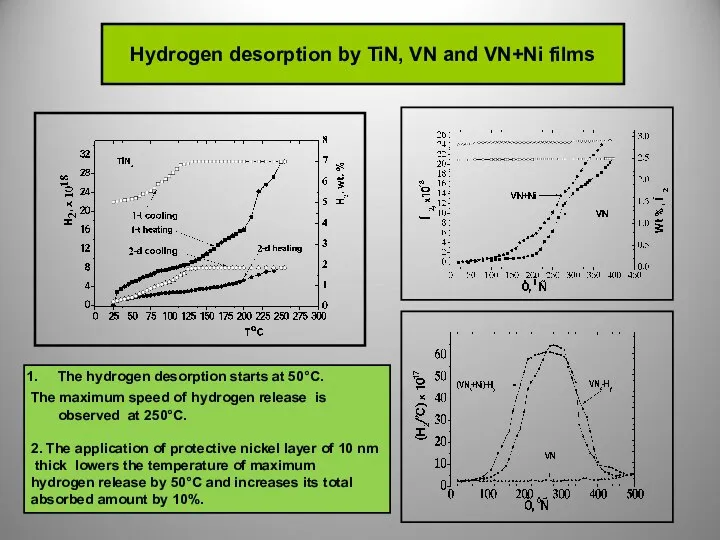
So far none of the solid-state hydrogen accumulators satisfy
the necessary requirements.





 Задания на ЕГЭ
Задания на ЕГЭ Аттестационная работа. Образовательная программа элективного курса Химия вокруг нас
Аттестационная работа. Образовательная программа элективного курса Химия вокруг нас Классификация химических веществ СГС
Классификация химических веществ СГС Биохимия почвообразования. Кора выветривания. Круговорот веществ в природе. Гранулометрический состав почвы. (Лекция 2)
Биохимия почвообразования. Кора выветривания. Круговорот веществ в природе. Гранулометрический состав почвы. (Лекция 2) Глюкоза. Загальна схема виробництва цукру. Підготували Учениці 9-б класу Василенко Алла Коваленко Катя Сердюк Яна
Глюкоза. Загальна схема виробництва цукру. Підготували Учениці 9-б класу Василенко Алла Коваленко Катя Сердюк Яна  Презентация по Химии "Малахіт" - скачать смотреть
Презентация по Химии "Малахіт" - скачать смотреть  Реакции, которые необходимо знать выпускнику средней школы, сдающему ЕГЭ по химии
Реакции, которые необходимо знать выпускнику средней школы, сдающему ЕГЭ по химии Гетероциклді қосылыстар. Алкалоидтар
Гетероциклді қосылыстар. Алкалоидтар Непрерывные реакторы для гомогенных гомофазных процессов
Непрерывные реакторы для гомогенных гомофазных процессов Циклоалканы. Лекция 4
Циклоалканы. Лекция 4 Муравьиная и уксусная кислоты
Муравьиная и уксусная кислоты Dzeramā ūdens sagatavošanas shēma
Dzeramā ūdens sagatavošanas shēma Презентация по Химии "Соединения серы (11 класс)" - скачать смотреть
Презентация по Химии "Соединения серы (11 класс)" - скачать смотреть  Технология производства и свойства искусственных волокон
Технология производства и свойства искусственных волокон Уголь. Виды угля
Уголь. Виды угля Окислительно-восстановительные процессы. Лекция 8
Окислительно-восстановительные процессы. Лекция 8 Фосфор и его соединения
Фосфор и его соединения Кислотність твердих тіл. Основні методи дослідження. Суперкислотність
Кислотність твердих тіл. Основні методи дослідження. Суперкислотність Презентация по Химии "Железо" - скачать смотреть бесплатно
Презентация по Химии "Железо" - скачать смотреть бесплатно Хімія числа Е в продуктах харчування
Хімія числа Е в продуктах харчування  Презентация по Химии "Фосфорные удобрения" - скачать смотреть бесплатно
Презентация по Химии "Фосфорные удобрения" - скачать смотреть бесплатно Углеводы. Классификация углеводов
Углеводы. Классификация углеводов Химия элементов VIIIA группы
Химия элементов VIIIA группы Органическая химия
Органическая химия Ионные уравнения
Ионные уравнения Reactions and equations lab
Reactions and equations lab Углерод и его соединения
Углерод и его соединения Коррозия металлов Учитель химии: Ильязова Р. Т.
Коррозия металлов Учитель химии: Ильязова Р. Т.