Содержание
- 2. Gastric cancer encompasses a heterogeneous collection of etiologic and histologic subtypes associated with a variety of
- 3. Approximately 3% to 5% of gastric cancers are associated with a hereditary predisposition, including a variety
- 4. Gastric cancer has traditionally been subtyped pathologically according to Lauren’s1 classification published in 1965 and revised
- 5. More clinically relevant, the majority of gastric cancers can be subdivided into intestinal type or diffuse
- 6. ETIOLOGY Environmental Risk Factors diet and lifestyle variables. Infectious Risk Factors H. pylori infection Epstein-Barr virus
- 7. More than 70% of cases occur in developing countries, and men have roughly twice the risk
- 8. PATHOLOGY AND TUMOR BIOLOGY Approximately 95% of all gastric cancers are adenocarcinomas.
- 9. PATTERNS OF SPREAD Carcinomas of the stomach can spread by local extension to involve adjacent structures
- 10. CLINICAL PRESENTATION AND PRETREATMENT EVALUATION Because of the vague, nonspecific symptoms that characterize gastric cancer, many
- 11. Up to 25% of the patients have history/symptoms of peptic ulcer disease. A history of dysphagia
- 12. PRETREATMENT STAGING Tumor markers – CEA, CA19-9,CA125 EUS CT MRI PET-CT Staging Laparoscopy and Peritoneal Cytology
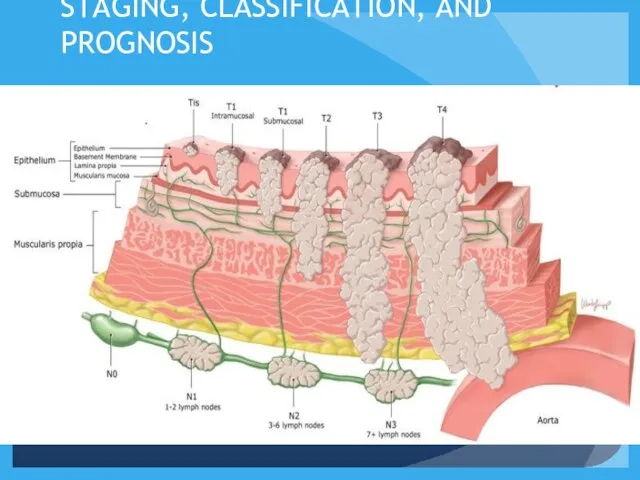
- 13. STAGING, CLASSIFICATION, AND PROGNOSIS
- 14. TREATMENT OF LOCALIZED DISEASE Stage I Disease (Early Gastric Cancer) Endoscopic Mucosal Resection Limited Surgical Resection
- 15. Stage II and Stage III Disease GASTRECTOMY
- 16. Adjuvant Therapy Adjuvant therapy indicates administration of a treatment following a potential curative resection of the
- 17. There are several theoretical reasons for beginning adjuvant therapy soon after operation (perioperative chemotherapy). Studies have
- 18. Neoadjuvant chemotherapy has a dual goal: allowing a higher rate of R0 resections and treatment of
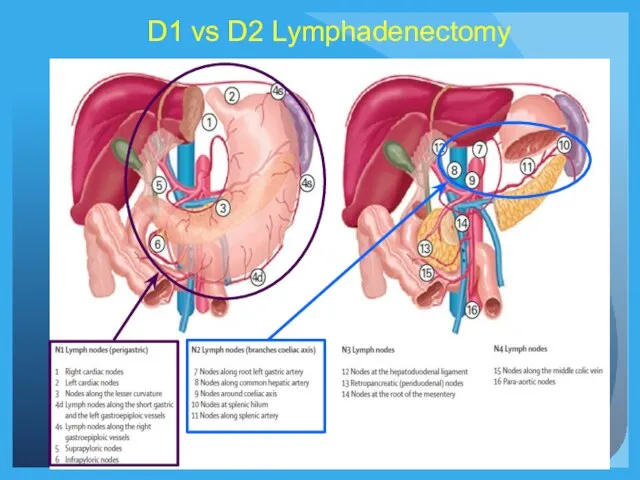
- 19. D1 vs D2 Lymphadenectomy
- 20. Rationale for Preoperative Therapy in Proximal Gastric Cancer Studies demonstrating benefit of preoperative chemotherapy over surgery
- 21. Importance of Preoperative Staging When Considering Neoadjuvant Therapy Accuracy of predicting nodal involvement is 60-80% Surgery
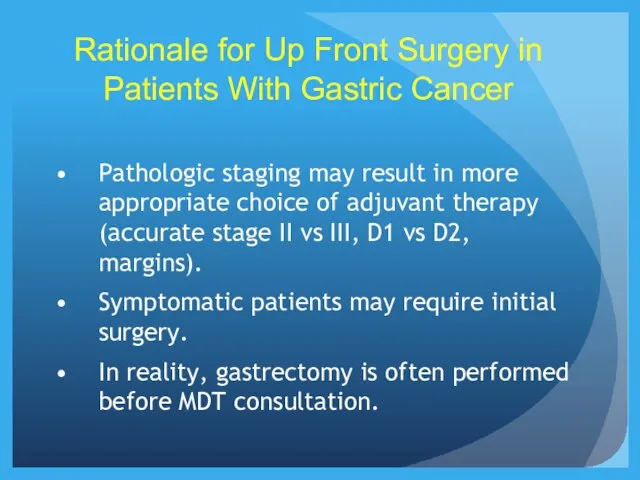
- 22. Rationale for Up Front Surgery in Patients With Gastric Cancer Pathologic staging may result in more
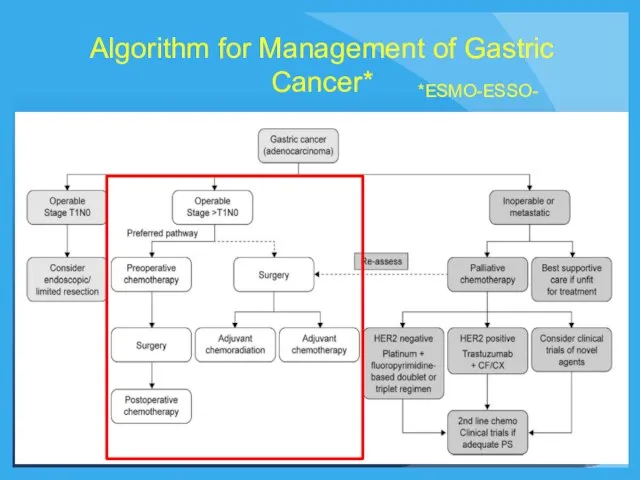
- 23. Algorithm for Management of Gastric Cancer* *ESMO-ESSO-
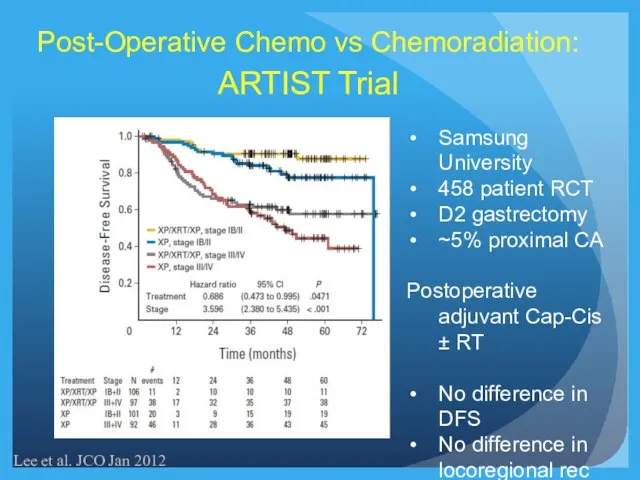
- 24. Post-Operative Chemo vs Chemoradiation: ARTIST Trial Lee et al. JCO Jan 2012 Samsung University 458 patient
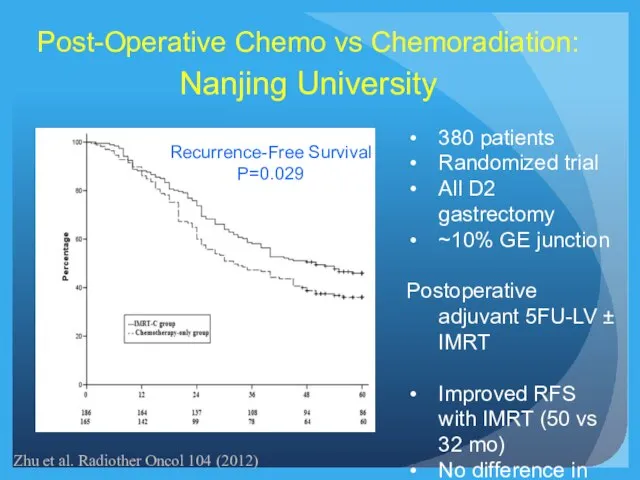
- 25. Recurrence-Free Survival P=0.029 Post-Operative Chemo vs Chemoradiation: Nanjing University 380 patients Randomized trial All D2 gastrectomy
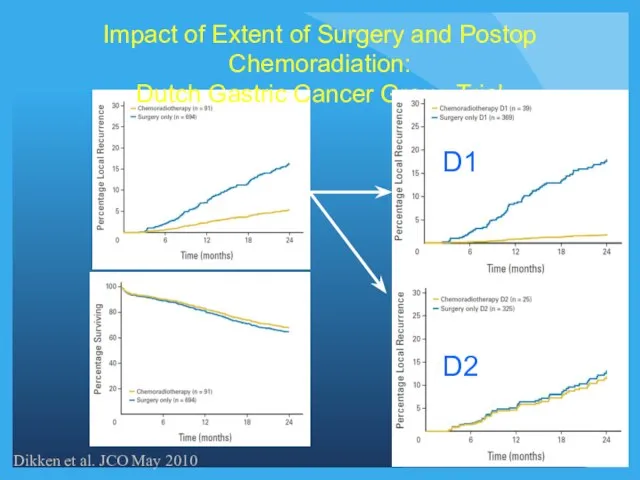
- 26. Impact of Extent of Surgery and Postop Chemoradiation: Dutch Gastric Cancer Group Trial Dikken et al.
- 27. MacDonald et al. NEJM 2001 Chemoradiation After Surgery Versus Surgery Alone for Gastric and GEJ Adenocarcinoma
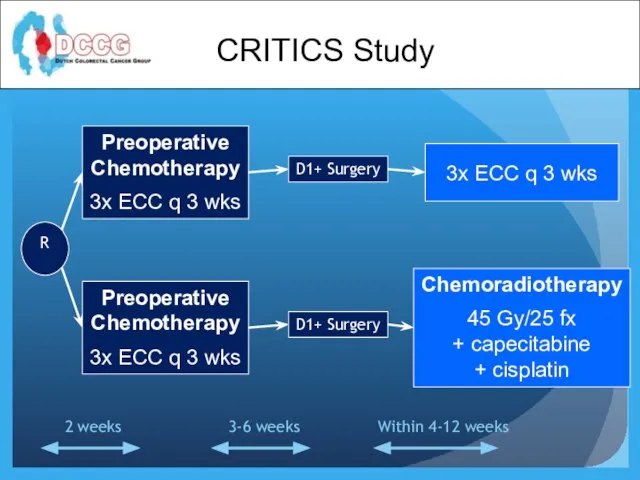
- 28. Preoperative Chemotherapy 3x ECC q 3 wks Preoperative Chemotherapy 3x ECC q 3 wks D1+ Surgery
- 29. Summary Adjuvant Therapy for Proximal Gastric Cancer While preoperative therapy may be preferred in most cases,
- 31. Скачать презентацию




















 Возбудители инфекций верхних дыхательных путей, характеризующихся специфичностью патогенеза и клинической картины
Возбудители инфекций верхних дыхательных путей, характеризующихся специфичностью патогенеза и клинической картины Сосудисто – тромбоцитарный гемостаз
Сосудисто – тромбоцитарный гемостаз Балалардағы тіс жарып шыққанға дейінгі тістің қатты тіндерінің тіс жегі емес ақауларының пайда болуы себептері
Балалардағы тіс жарып шыққанға дейінгі тістің қатты тіндерінің тіс жегі емес ақауларының пайда болуы себептері Темперамент. Типы темперамента и их психологическая характеристика
Темперамент. Типы темперамента и их психологическая характеристика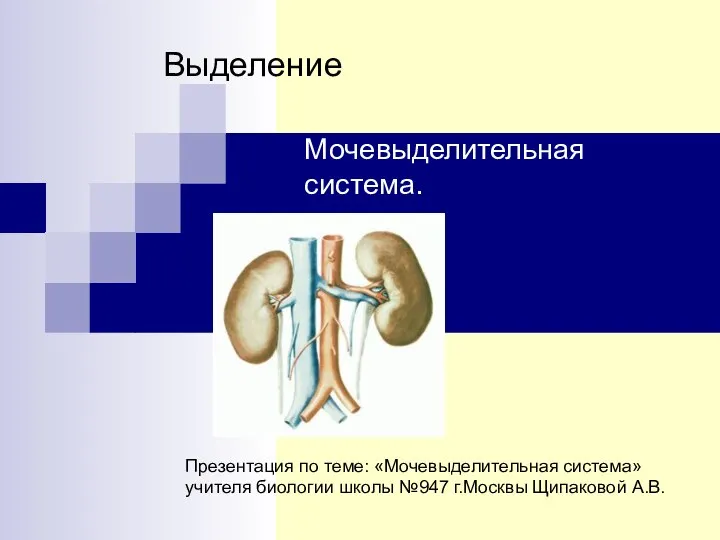 Мочевыделительная система
Мочевыделительная система Сам себе косметолог. Уход за кожей лица
Сам себе косметолог. Уход за кожей лица Орталық және вегетативті нерв жүйесінің клиникалық физиологиясы және биохимиясы
Орталық және вегетативті нерв жүйесінің клиникалық физиологиясы және биохимиясы Сосудистые и пигментные дефекты
Сосудистые и пигментные дефекты Кардиотокография (КТГ)
Кардиотокография (КТГ) Рентгенологические методы диагностики в стоматологии
Рентгенологические методы диагностики в стоматологии Эффекты, возникающие при повторном введении лекарственных веществ. Взаимодействие лекарств
Эффекты, возникающие при повторном введении лекарственных веществ. Взаимодействие лекарств Послеродовый гипопитуитаризм (синдром Шихана)
Послеродовый гипопитуитаризм (синдром Шихана) Буллинг. Типы буллинга
Буллинг. Типы буллинга Ветряная оспа
Ветряная оспа Иерсиниозные инфекции, иерсиниозы
Иерсиниозные инфекции, иерсиниозы Опухоли
Опухоли Асептика. История развития хирургии
Асептика. История развития хирургии Важность правильного питания
Важность правильного питания Лимфоциты. Мембранные маркеры лимфоцитов
Лимфоциты. Мембранные маркеры лимфоцитов Мутацияның молекулалық негіздері
Мутацияның молекулалық негіздері Дәрігермен науқас арасындағы қарым-қатынас түрлерін қолдану
Дәрігермен науқас арасындағы қарым-қатынас түрлерін қолдану Интервенционная радиология в онкологии
Интервенционная радиология в онкологии Толпа
Толпа Микроорганизмы - возбудители антропозоонозных инфекций
Микроорганизмы - возбудители антропозоонозных инфекций Особенности фармакотерапии неотложных состояний
Особенности фармакотерапии неотложных состояний Дифференциальная диагностика алопеции (выпадения волос) в фазе телогена
Дифференциальная диагностика алопеции (выпадения волос) в фазе телогена Травмофокусована когнітивно-поведінкова терапія посттравматичного стресового розладу
Травмофокусована когнітивно-поведінкова терапія посттравматичного стресового розладу Септические осложнение послеабортного периода
Септические осложнение послеабортного периода