Содержание
- 2. The names are: Mushroom, blind, blood, glazed rains. And still there is one particular one the
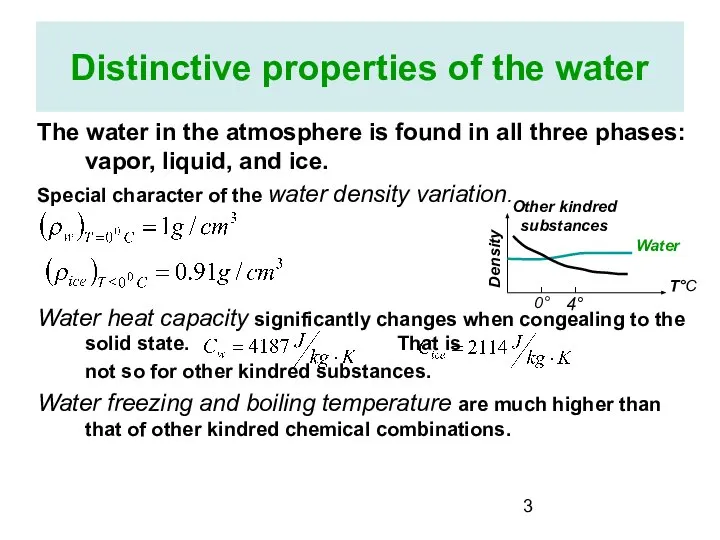
- 3. Distinctive properties of the water The water in the atmosphere is found in all three phases:
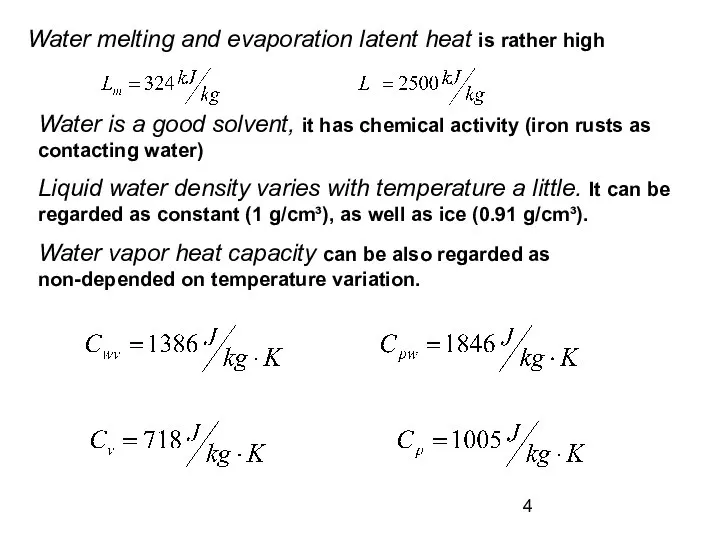
- 4. Water melting and evaporation latent heat is rather high Water is a good solvent, it has
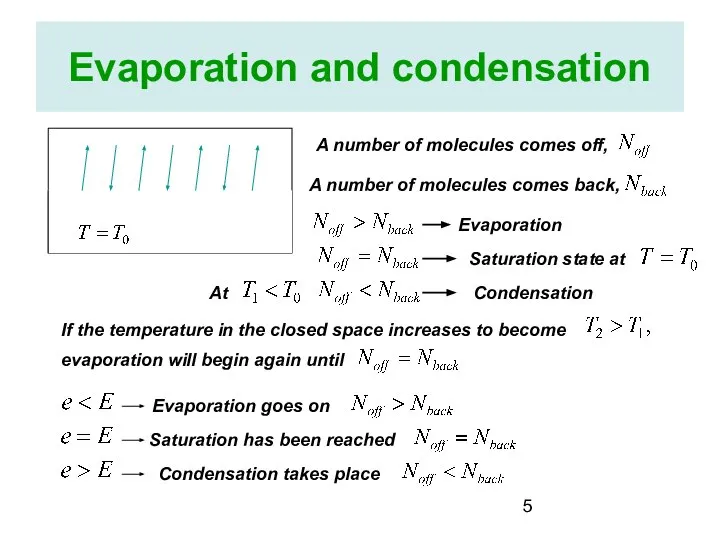
- 5. Evaporation and condensation A number of molecules comes off, A number of molecules comes back, Evaporation
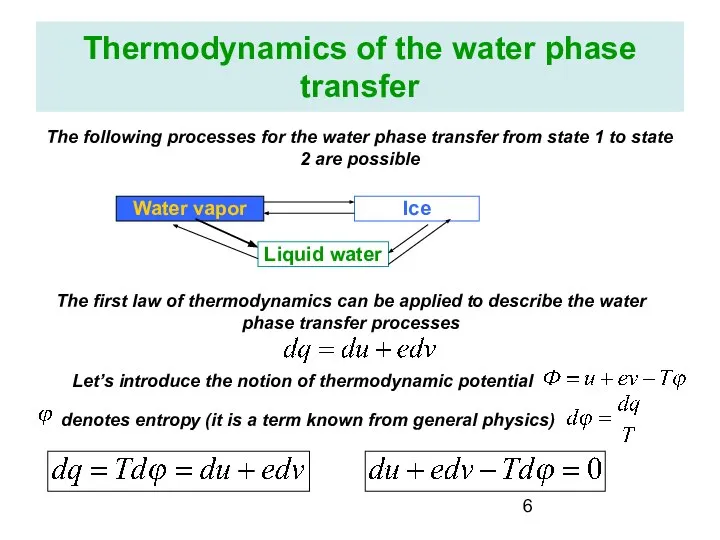
- 6. Thermodynamics of the water phase transfer The following processes for the water phase transfer from state
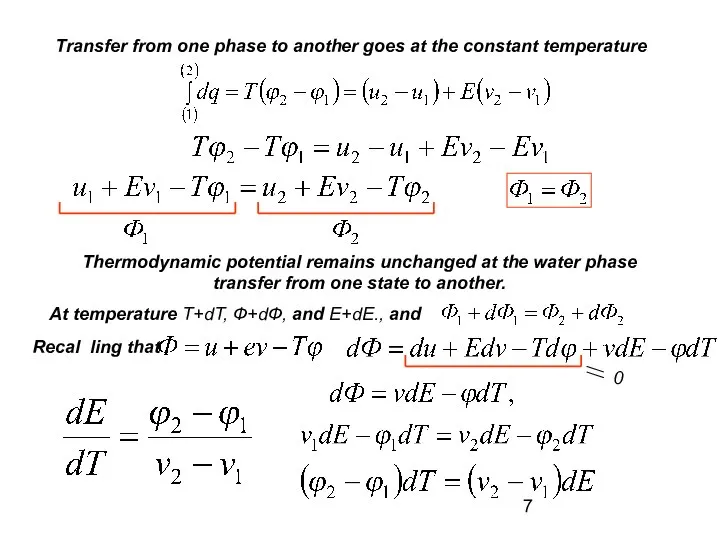
- 7. Transfer from one phase to another goes at the constant temperature Thermodynamic potential remains unchanged at
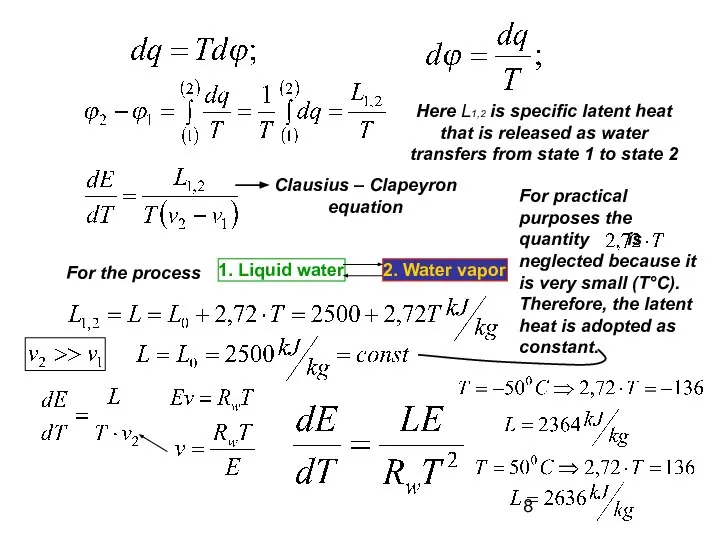
- 8. Here L1,2 is specific latent heat that is released as water transfers from state 1 to
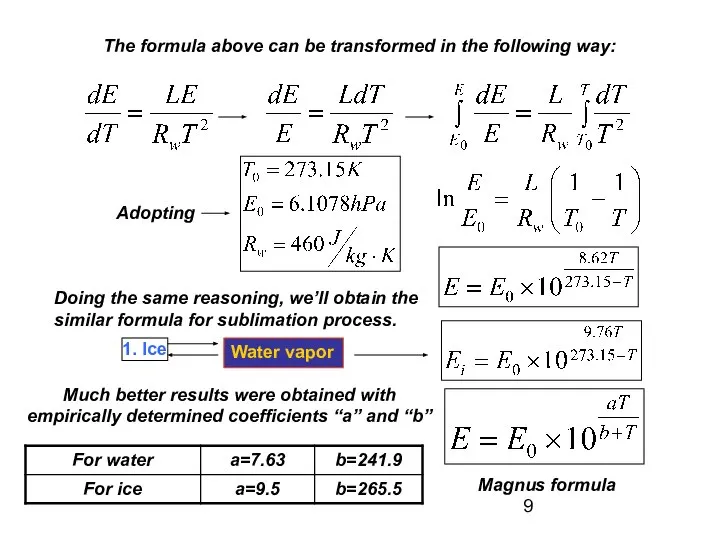
- 9. The formula above can be transformed in the following way: Adopting Doing the same reasoning, we’ll
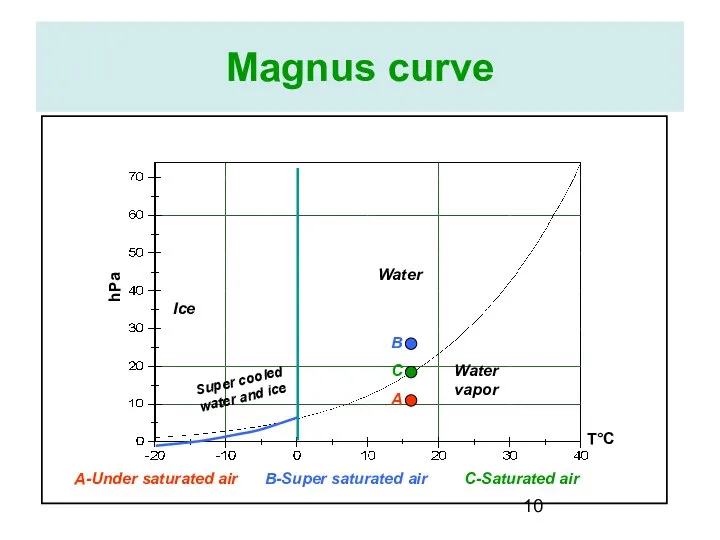
- 10. Magnus curve Water Ice Water vapor Super cooled water and ice T°C hPa A B C
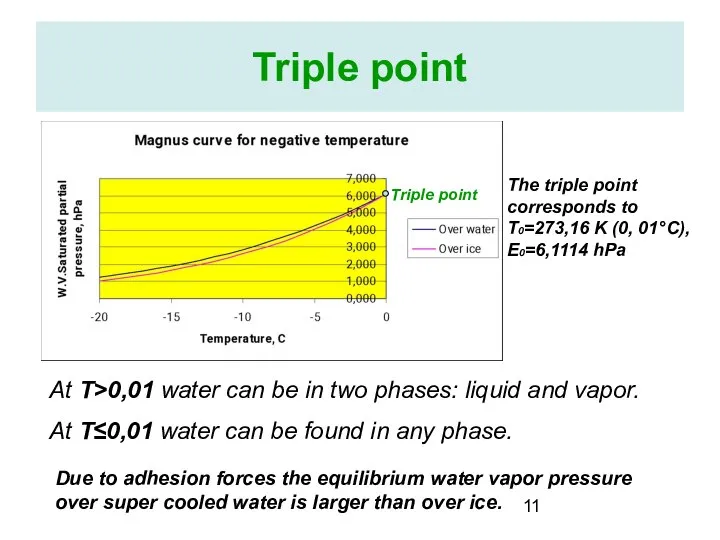
- 11. Triple point Triple point The triple point corresponds to T0=273,16 K (0, 01°C), E0=6,1114 hPa At
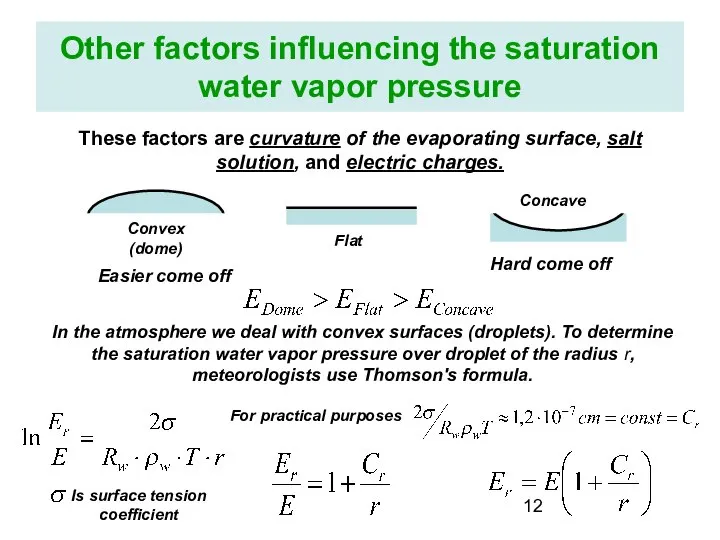
- 12. Other factors influencing the saturation water vapor pressure These factors are curvature of the evaporating surface,
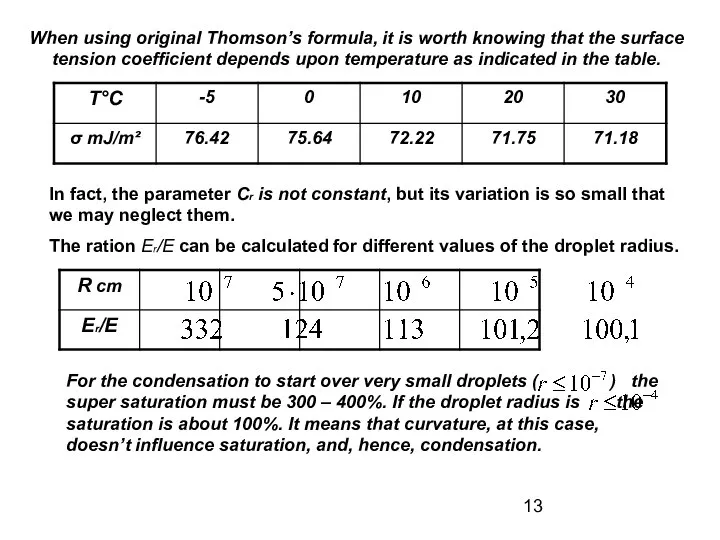
- 13. When using original Thomson’s formula, it is worth knowing that the surface tension coefficient depends upon
- 15. Скачать презентацию

 Роль комплексного анализа в управлении
Роль комплексного анализа в управлении Правила назначения и отпуска лекарственных препаратов
Правила назначения и отпуска лекарственных препаратов Права человека
Права человека «Внедрение компьютерной технологии в учебную деятельность по русскому языку и литературе». «Внедрение компьютерной технологии
«Внедрение компьютерной технологии в учебную деятельность по русскому языку и литературе». «Внедрение компьютерной технологии  Humour Rules
Humour Rules Строительные материалы
Строительные материалы Глобальні проблеми людства
Глобальні проблеми людства Статистика туризма Казахстана
Статистика туризма Казахстана Электрические и магнитные элементы автоматики
Электрические и магнитные элементы автоматики Худеем вместе
Худеем вместе Измерение сезонных колебаний
Измерение сезонных колебаний Волейбол
Волейбол Джеффри Лайкер «Дао Toyota: 14 принципов менеджмента ведущей компании мира»
Джеффри Лайкер «Дао Toyota: 14 принципов менеджмента ведущей компании мира»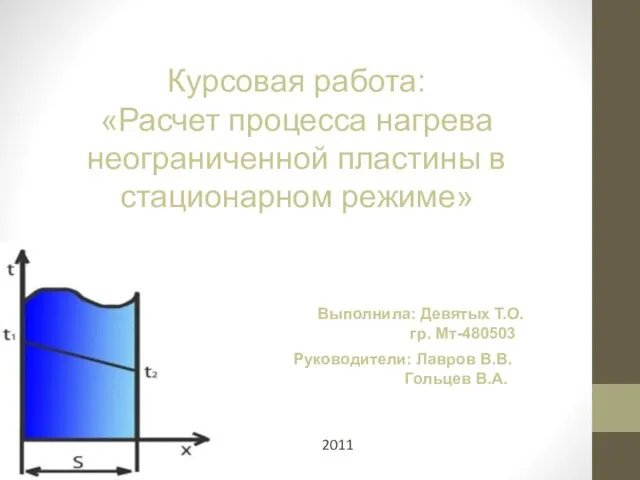 20112011Нагрев (охлаждение) неограниченной пластины ведется в регулярном режиме. Регулярный режим - режим, который начинается с некото
20112011Нагрев (охлаждение) неограниченной пластины ведется в регулярном режиме. Регулярный режим - режим, который начинается с некото ПОСТРОЕНИЕ ДИАГРАММ В табличном процессоре Microsoft Excel
ПОСТРОЕНИЕ ДИАГРАММ В табличном процессоре Microsoft Excel Суть и назначение системного анализа как методологической основы анализа
Суть и назначение системного анализа как методологической основы анализа Подготовила: Порошина Л.В., студентка 4 курса юридического факультета РТА, гр. Ю102 _
Подготовила: Порошина Л.В., студентка 4 курса юридического факультета РТА, гр. Ю102 _ Четыре измерения медиаконвергенции
Четыре измерения медиаконвергенции European law
European law Исследовательская программа Ф.А. фон Хайека. Лекция Н.А. Макашевой для 2-ого курса
Исследовательская программа Ф.А. фон Хайека. Лекция Н.А. Макашевой для 2-ого курса  Презентация "Жан Батист Мольер" - скачать презентации по МХК
Презентация "Жан Батист Мольер" - скачать презентации по МХК ПРИКАЗ от 29 января 2008 г. N 80 ВОПРОСЫ ОРГАНИЗАЦИИ ДЕЯТЕЛЬНОСТИ СТРОЕВЫХ ПОДРАЗДЕЛЕНИЙ ПАТРУЛЬНО-ПОСТОВОЙ СЛУЖБЫ ПОЛИЦИИ В целях сов
ПРИКАЗ от 29 января 2008 г. N 80 ВОПРОСЫ ОРГАНИЗАЦИИ ДЕЯТЕЛЬНОСТИ СТРОЕВЫХ ПОДРАЗДЕЛЕНИЙ ПАТРУЛЬНО-ПОСТОВОЙ СЛУЖБЫ ПОЛИЦИИ В целях сов Изучение и использование мемристоров в качестве революционного прикладного применения
Изучение и использование мемристоров в качестве революционного прикладного применения Социология политики и управления. (Тема 10)
Социология политики и управления. (Тема 10) Роботизированная коробка передач DSG
Роботизированная коробка передач DSG Biografy of Sergey Bezrykov
Biografy of Sergey Bezrykov Государственная политика в сфере информатизации общества и информационной безопасности
Государственная политика в сфере информатизации общества и информационной безопасности Новоассирийская и нововавилонская державы
Новоассирийская и нововавилонская державы