Содержание
- 2. INTRODUCTION Name of this family comes from the properties of alkali metals to form hydroxides with
- 3. GENERAL PROPERTIES OF 1A *By giving their valence electron easily in chemical reactions, they form +1
- 4. They are solids at room temperature. They are soft. They can be cut by a knife.
- 5. OCCURRENCE Since the alkali metals are the most active metals, they are not found free in
- 6. Potassium, K Potassium constitutes 1.5% of the earth’s crust. Potassium is found as the minerals sylvite
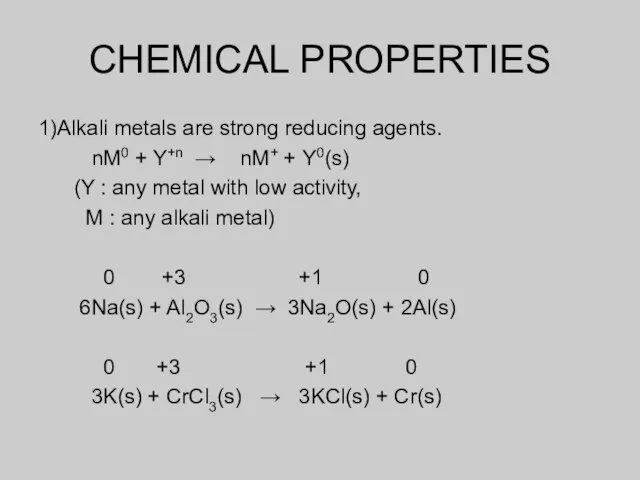
- 7. CHEMICAL PROPERTIES 1)Alkali metals are strong reducing agents. nM0 + Y+n → nM+ + Y0(s) (Y
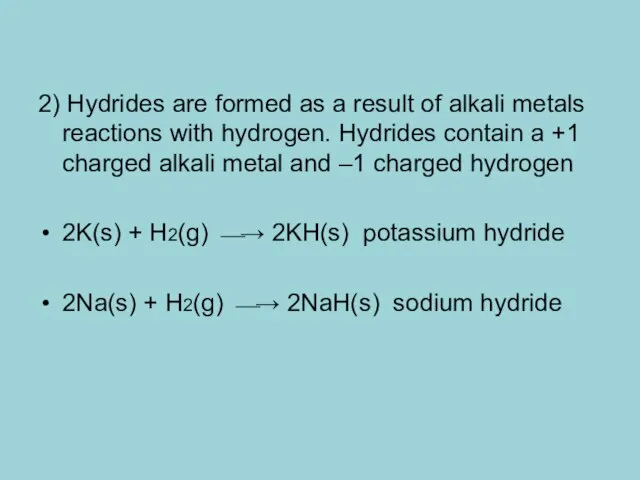
- 8. 2) Hydrides are formed as a result of alkali metals reactions with hydrogen. Hydrides contain a
- 9. 3) They react with water violently. As a result of this reaction H2 gas and a
- 10. 4) They may form oxides, peroxides or superoxides by reacting with oxygen in the air. As
- 11. 5. All of them react with halogens to form alkali halides (salts of alkali metals). 2M(s)
- 12. 6. They do not react with bases M(s) + OH–(aq) ⎯→ No reaction 7. When they
- 14. Скачать презентацию









 1_Bazovye_raschetnye_formuly
1_Bazovye_raschetnye_formuly Гетерофункциональные органические соединения
Гетерофункциональные органические соединения Свойства катализатора. Влияние массы катализатора на скорость реакции
Свойства катализатора. Влияние массы катализатора на скорость реакции Отбор проб товаров для анализа. Химико-аналитический контроль
Отбор проб товаров для анализа. Химико-аналитический контроль Сопряженное присоединение. Циклоприсоединениеie
Сопряженное присоединение. Циклоприсоединениеie Масс-спектрометрия (МС)
Масс-спектрометрия (МС) Классификации, номенклатура, строение и свойства органических соединений
Классификации, номенклатура, строение и свойства органических соединений Великие ученые XIX-XX веков
Великие ученые XIX-XX веков Презентация Электролиты
Презентация Электролиты Атоми і хімічні елементи. Молекули, їх рух. Дифузія
Атоми і хімічні елементи. Молекули, їх рух. Дифузія Производство шампуня. Технология
Производство шампуня. Технология 140 лет дому, который построил Д.И. Менделеев
140 лет дому, который построил Д.И. Менделеев 160198375
160198375 Презентация Лекарства дома
Презентация Лекарства дома Биохимия. Введение
Биохимия. Введение Углеводы (моносахариды, олигосахариды, полисахариды)
Углеводы (моносахариды, олигосахариды, полисахариды) Автомобильные бензины
Автомобильные бензины Силикатная промышленность
Силикатная промышленность Кислоты
Кислоты Виробництво біогазу Виконав: студент IV курсу групи БЛБ-43з Вила Віктор
Виробництво біогазу Виконав: студент IV курсу групи БЛБ-43з Вила Віктор  Хроматографические методы анализа
Хроматографические методы анализа Электролиз
Электролиз Оксиды. Химические свойства
Оксиды. Химические свойства Основные законы химии. Законы и формулировки
Основные законы химии. Законы и формулировки Метод крутого восхождения или метод Бокса-Уилсона. Симплексный метод оптимизации
Метод крутого восхождения или метод Бокса-Уилсона. Симплексный метод оптимизации Окислительно-восстановительные реакции
Окислительно-восстановительные реакции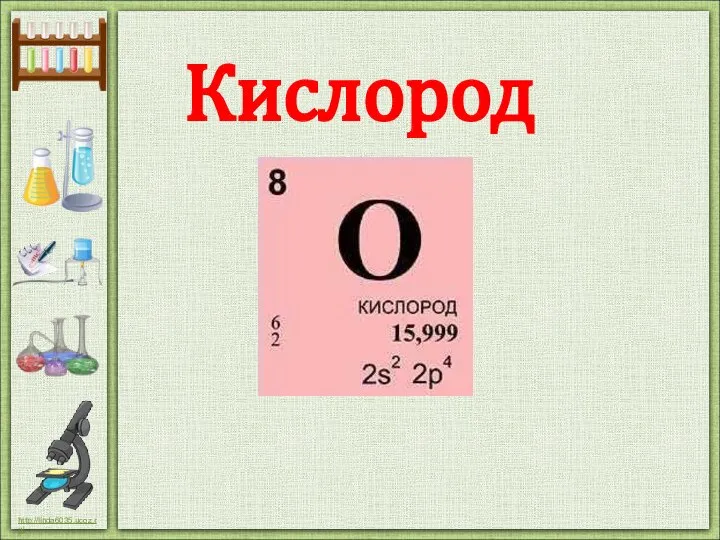 Кислород. Свойства кислорода
Кислород. Свойства кислорода Валентность химических элементов (8 класс)
Валентность химических элементов (8 класс)