Содержание
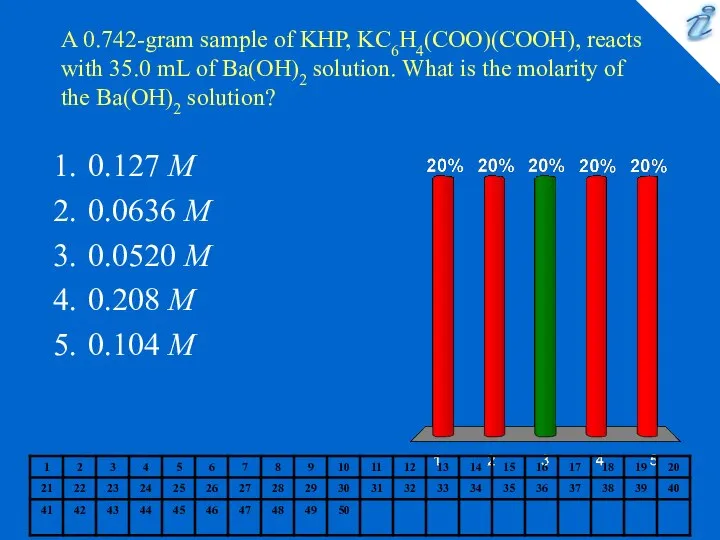
- 2. A 0.742-gram sample of KHP, KC6H4(COO)(COOH), reacts with 35.0 mL of Ba(OH)2 solution. What is the
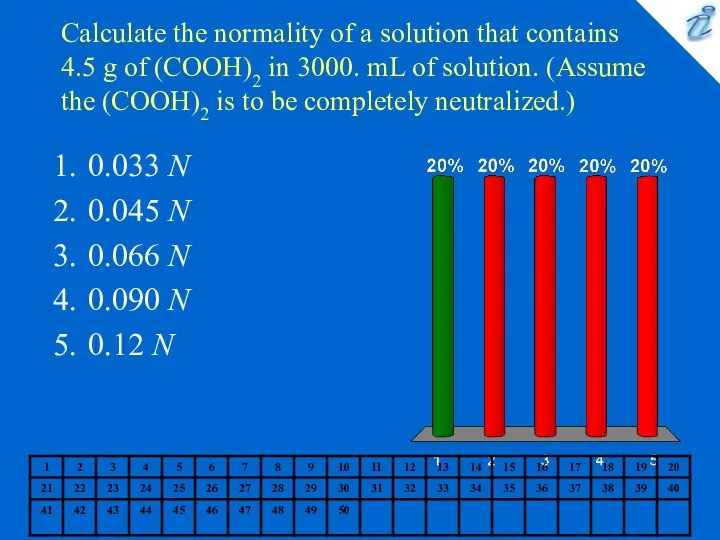
- 3. Calculate the normality of a solution that contains 4.5 g of (COOH)2 in 3000. mL of
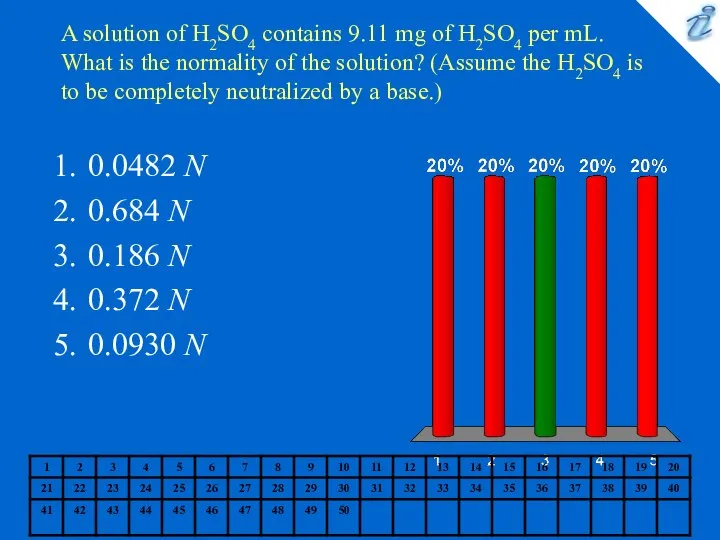
- 4. A solution of H2SO4 contains 9.11 mg of H2SO4 per mL. What is the normality of
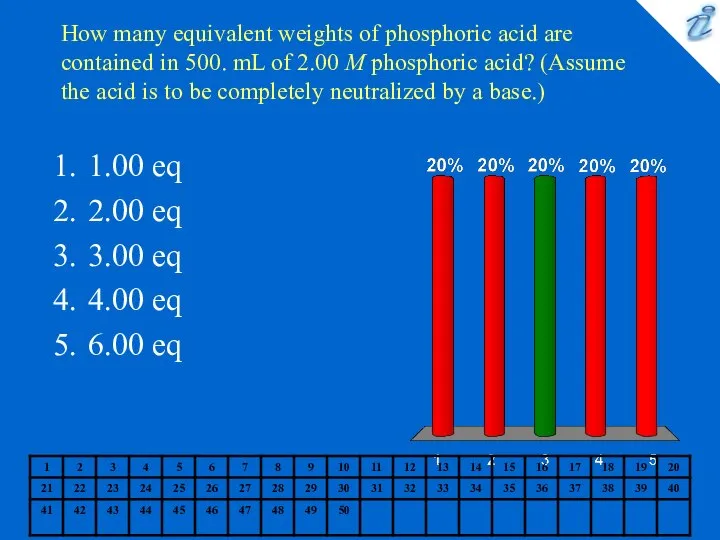
- 5. How many equivalent weights of phosphoric acid are contained in 500. mL of 2.00 M phosphoric
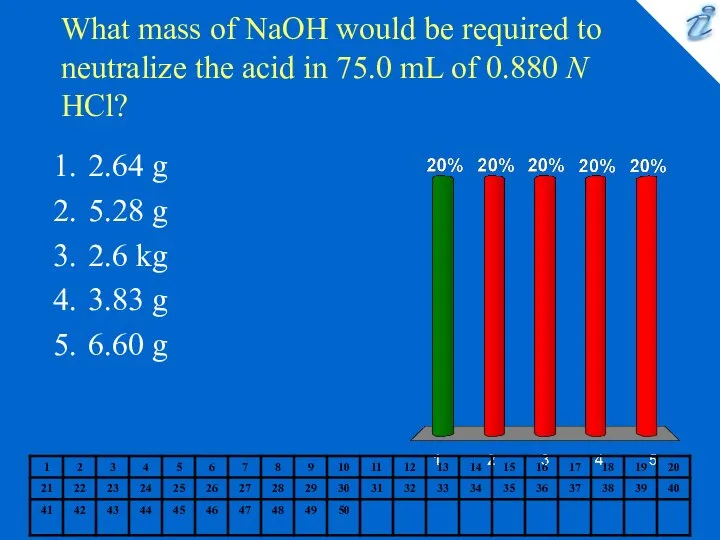
- 6. What mass of NaOH would be required to neutralize the acid in 75.0 mL of 0.880
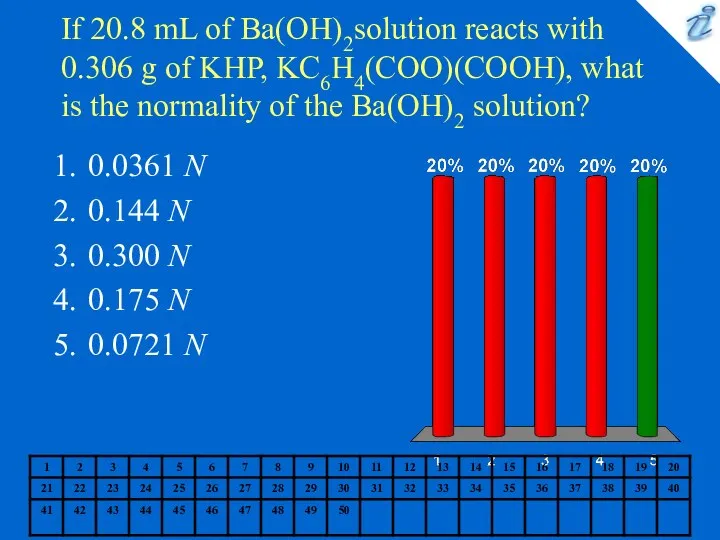
- 7. If 20.8 mL of Ba(OH)2solution reacts with 0.306 g of KHP, KC6H4(COO)(COOH), what is the normality
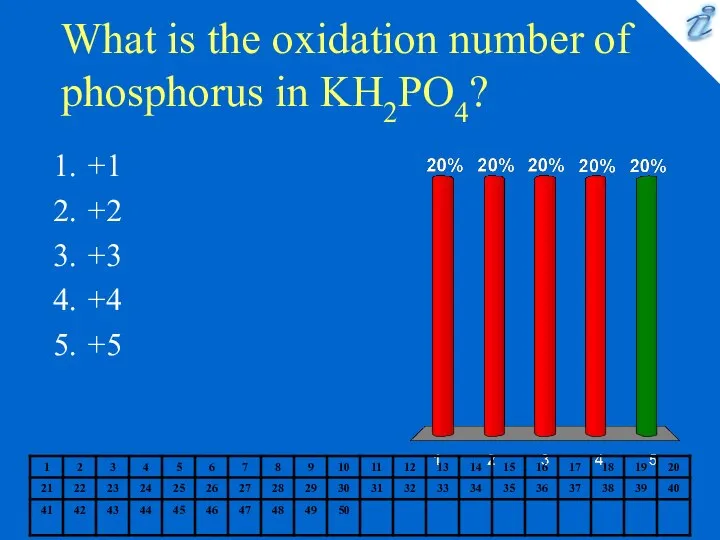
- 8. What is the oxidation number of phosphorus in KH2PO4? +1 +2 +3 +4 +5
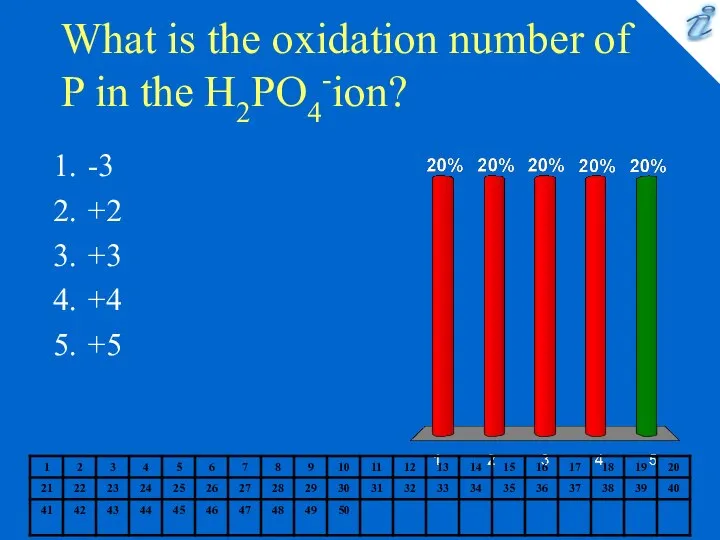
- 9. What is the oxidation number of P in the H2PO4-ion? -3 +2 +3 +4 +5
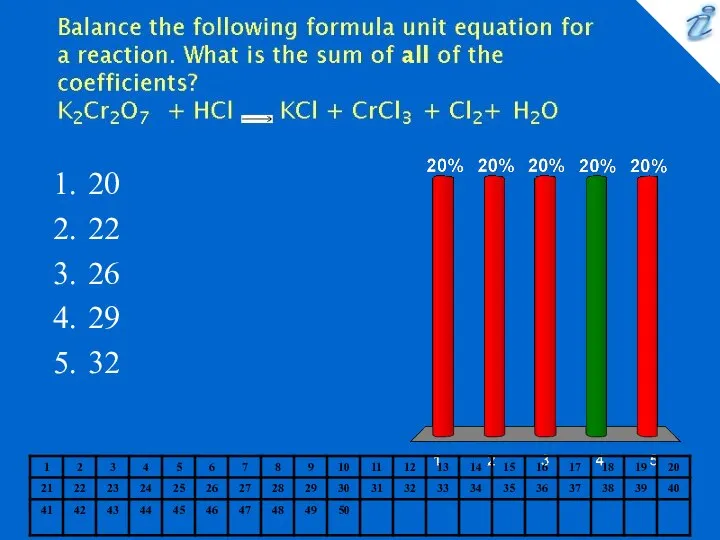
- 10. 20 22 26 29 32
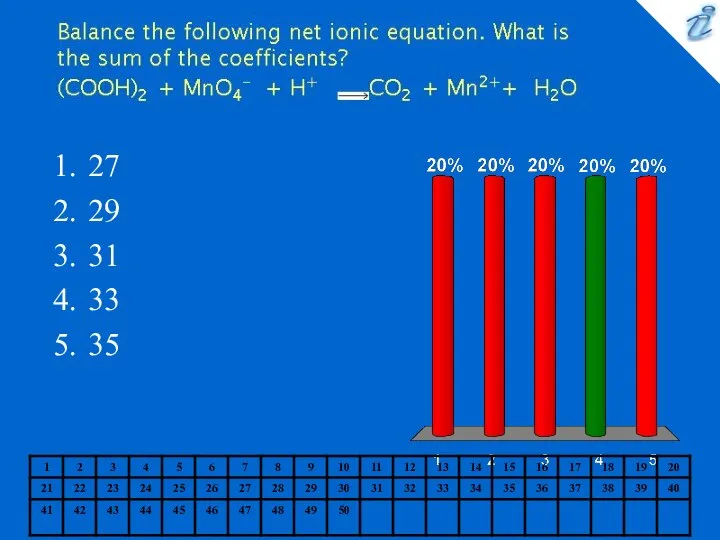
- 11. 27 29 31 33 35
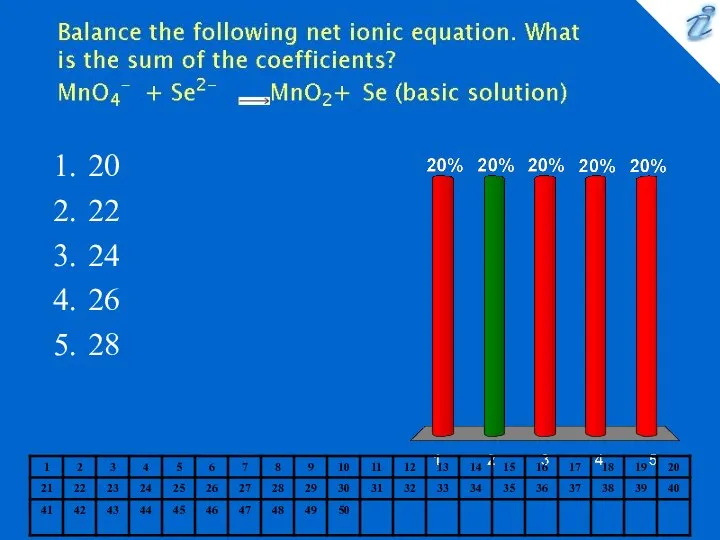
- 12. 20 22 24 26 28
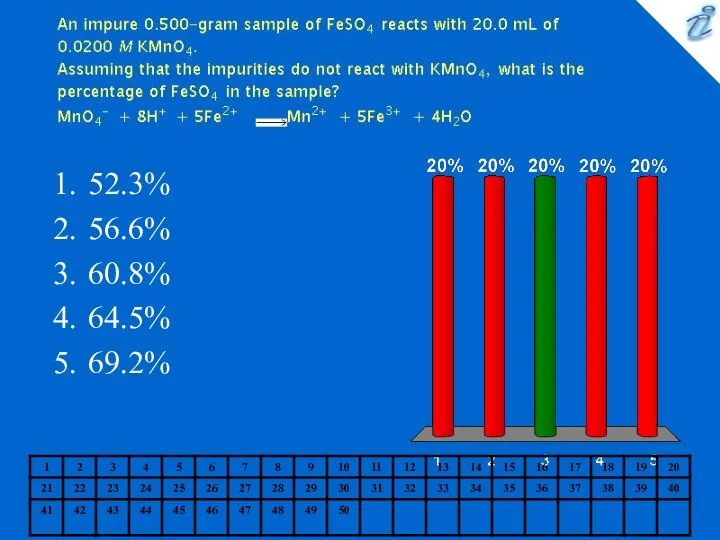
- 13. 52.3% 56.6% 60.8% 64.5% 69.2%
- 15. Скачать презентацию
 Производство геля для душа и его состав
Производство геля для душа и его состав Основные понятия и законы химии
Основные понятия и законы химии Что объединяет вещества. Классификация твёрдых веществ
Что объединяет вещества. Классификация твёрдых веществ Методические рекомендации к уроку по теме Скорость химических реакций в 11классе
Методические рекомендации к уроку по теме Скорость химических реакций в 11классе Железо и его соединения
Железо и его соединения Симметрия, структура и свойства твердых тел – кристаллография и кристаллофизика
Симметрия, структура и свойства твердых тел – кристаллография и кристаллофизика Стани води
Стани води Функціональні матеріали в аналітичній хімії. Лекція 1
Функціональні матеріали в аналітичній хімії. Лекція 1 Презентация по Химии "Кислородсодержащие органические вещества. Фенолы" - скачать смотреть
Презентация по Химии "Кислородсодержащие органические вещества. Фенолы" - скачать смотреть  Общая и неорганическая химия. Лекция 20 Особенности химии серы. Водородные и кислородные соединения
Общая и неорганическая химия. Лекция 20 Особенности химии серы. Водородные и кислородные соединения Витамины и коферменты
Витамины и коферменты Диаграмма состояния железо–углерод
Диаграмма состояния железо–углерод Перспективы использования оксигенатов в моторных топливах
Перспективы использования оксигенатов в моторных топливах Многоатомные спирты
Многоатомные спирты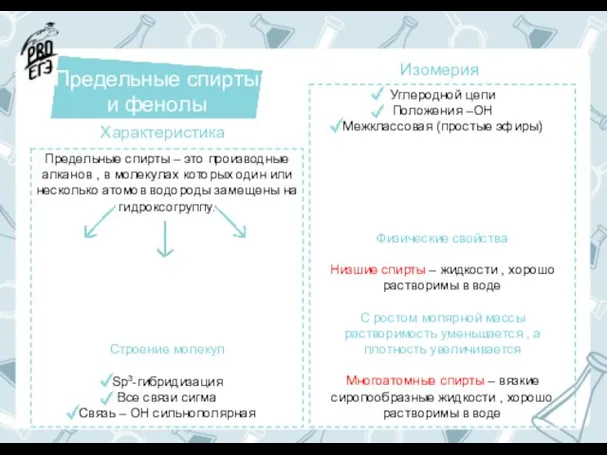 Предельные спирты и фенолы
Предельные спирты и фенолы Повышение огнестойкости деревянных конструкций методом поверхностной и объёмной пропитки
Повышение огнестойкости деревянных конструкций методом поверхностной и объёмной пропитки Соединения щелочноземельных металлов
Соединения щелочноземельных металлов Характеристика химического элемента на основании его положения в Периодической системе Д.И. Менделеева (урок 1 - 2)
Характеристика химического элемента на основании его положения в Периодической системе Д.И. Менделеева (урок 1 - 2) Пестицидтердің қоршаған ортаға зиян-ын тигізбеу жүйесі. Пестицидтерді қолданудың физикалық-химиялық негіздері
Пестицидтердің қоршаған ортаға зиян-ын тигізбеу жүйесі. Пестицидтерді қолданудың физикалық-химиялық негіздері Качественные реакции на функциональные группы
Качественные реакции на функциональные группы Строение и свойства углеводов и липидов
Строение и свойства углеводов и липидов КОВАЛЕНТНАЯ СВЯЗЬ ПОЛЯРНАЯ И НЕПОЛЯРНАЯ
КОВАЛЕНТНАЯ СВЯЗЬ ПОЛЯРНАЯ И НЕПОЛЯРНАЯ УГЛЕВОДЫ И ЛИПИДЫ. ИХ РОЛЬ В ЖИЗНЕДЕЯТЕЛЬНОСТИ КЛЕТКИ Презентация учителя химии, биологии и экологии МОУ СОШ п.Алексеевка Беша
УГЛЕВОДЫ И ЛИПИДЫ. ИХ РОЛЬ В ЖИЗНЕДЕЯТЕЛЬНОСТИ КЛЕТКИ Презентация учителя химии, биологии и экологии МОУ СОШ п.Алексеевка Беша Дәнді-дақылдар тыңайтқыштары
Дәнді-дақылдар тыңайтқыштары Тепловой эффект сгорания топлива
Тепловой эффект сгорания топлива Предмет и метод термодинамики. Химическая термодинамика
Предмет и метод термодинамики. Химическая термодинамика Лягушачье золото
Лягушачье золото Оценка солености воды реки Цны и питьевой бутилированной воды
Оценка солености воды реки Цны и питьевой бутилированной воды