Содержание
- 2. Formula O2
- 3. Transcription ['ɔksiʤ(ə)n]
- 4. Etymology It is derived from Greek and it means «acid former».
- 5. Position in the periodic table Oxygen is in group 16 and period 2 of the Periodic
- 6. Нistory of discovery Oxygen was obtained by C. W. Scheele and J. Priestley independently.
- 7. Structure The oxygen molecule consists of two atoms. The mechanism of its formation is non-polar covalent.
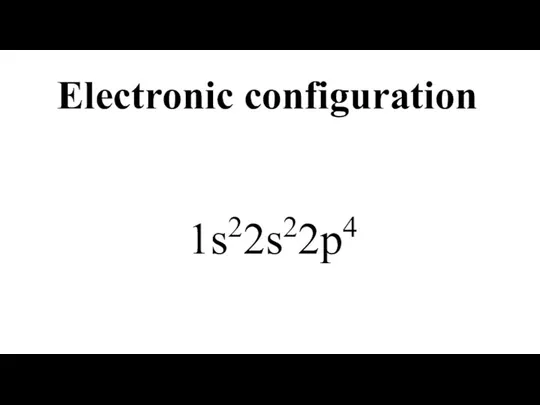
- 8. Electronic configuration 1s22s22p4
- 9. Atomic mass 16 grams per mole
- 10. Radius of the oxygen atom 60
- 11. Valence –2, −1, –½, –⅓, 0, +½, +1, +2
- 12. Boiling/melting point The melting point is 54.8 K (-218.35 °C). The boiling point is 90.19 K
- 13. Isotopes Oxygen-16 Oxygen-17 Oxygen-18
- 14. Physical properties Oxygen is the gas. Oxygen has no color, no taste, and no smell. Oxygen
- 15. Prevalence in nature It forms 21 % of the atmosphere, 89 % 0f the water, 50%
- 16. Оxygen deposit It occurs in the atmosphere, in water, in the earth’s crust.
- 17. Мethods of obtaining oxygen It is easily prepared in the laboratory by heating potassium chlorate. It
- 18. Сhemical properties Flammability - Does not burn; Combustion - Supports combustion but does not burn; Compounds
- 19. Application in industry Oxygen is actively used in: Metallurgy (during welding and cutting metals). The medicine.
- 20. The biological role The presence of oxygen (in combination with water) has made life possible on
- 22. Скачать презентацию

![Transcription ['ɔksiʤ(ə)n]](/_ipx/f_webp&q_80&fit_contain&s_1440x1080/imagesDir/jpg/706599/slide-2.jpg)
















 Спирти та їх види
Спирти та їх види Подготовка к ГИА. Тренажер по химии
Подготовка к ГИА. Тренажер по химии Жесткость воды и способы ее устранения
Жесткость воды и способы ее устранения Презентация СПЛАВЫ МЕТАЛЛОВ
Презентация СПЛАВЫ МЕТАЛЛОВ  Отрицательное влияние азотных минеральных удобрений на почву
Отрицательное влияние азотных минеральных удобрений на почву Катализ органических реакций. (Лекция 15)
Катализ органических реакций. (Лекция 15) Термический анализ
Термический анализ Физическая и коллоидная химия
Физическая и коллоидная химия Технический контроль химических соединений в производстве каустика
Технический контроль химических соединений в производстве каустика Углеводороды. Алкены
Углеводороды. Алкены Продукты переработки жиров. Жироподобные вещества. Заменители жиров в косметическом производстве
Продукты переработки жиров. Жироподобные вещества. Заменители жиров в косметическом производстве Вакуумное фильтрование
Вакуумное фильтрование Введение в хемоинформатику
Введение в хемоинформатику Кремнийорганикалық қосылыстар
Кремнийорганикалық қосылыстар Контроль в процессе обучения химии на старшей ступени школы
Контроль в процессе обучения химии на старшей ступени школы Составление электронных формул и электронно-графических схем строения атома
Составление электронных формул и электронно-графических схем строения атома Урок повторения по химии за курс 8 класса
Урок повторения по химии за курс 8 класса Азотная кислота
Азотная кислота Анилин. Строение. Физические и химические свойства
Анилин. Строение. Физические и химические свойства Композиционные материалы, состав и классификация композиционных материалов
Композиционные материалы, состав и классификация композиционных материалов Простые вещества – металлы. Общие физические свойства металлов.
Простые вещества – металлы. Общие физические свойства металлов. Распространение ПВО в приземном слое атмосферы
Распространение ПВО в приземном слое атмосферы Строение атома. (Лекция 2-3)
Строение атома. (Лекция 2-3) Типы кристаллических решеток. Вещества молекулярного и немолекулярного строения
Типы кристаллических решеток. Вещества молекулярного и немолекулярного строения Веселый химический КВН
Веселый химический КВН Соли
Соли Горные породы
Горные породы Леция 1. Природа сил взаимодействия. Методы исследования комплексообразования:
Леция 1. Природа сил взаимодействия. Методы исследования комплексообразования: