Содержание
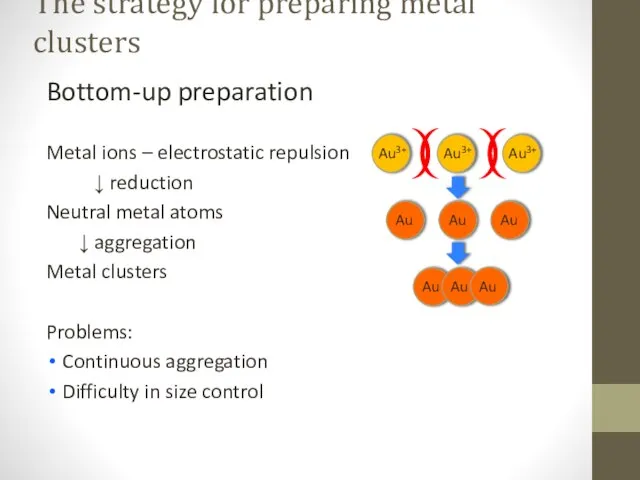
- 2. The strategy for preparing metal clusters Bottom-up preparation Metal ions – electrostatic repulsion ↓ reduction Neutral
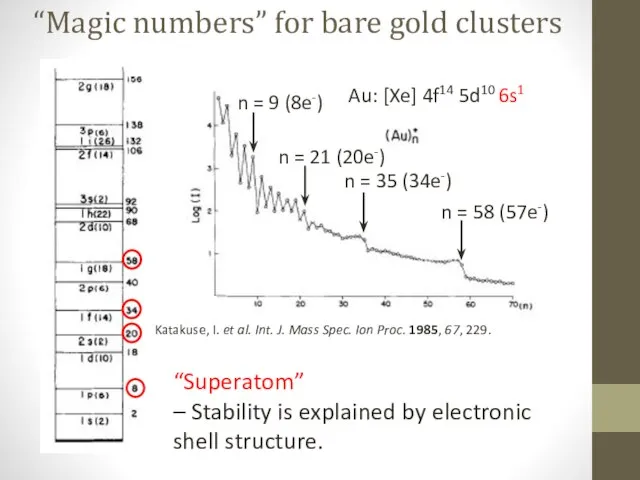
- 3. “Magic numbers” for bare gold clusters Katakuse, I. et al. Int. J. Mass Spec. Ion Proc.
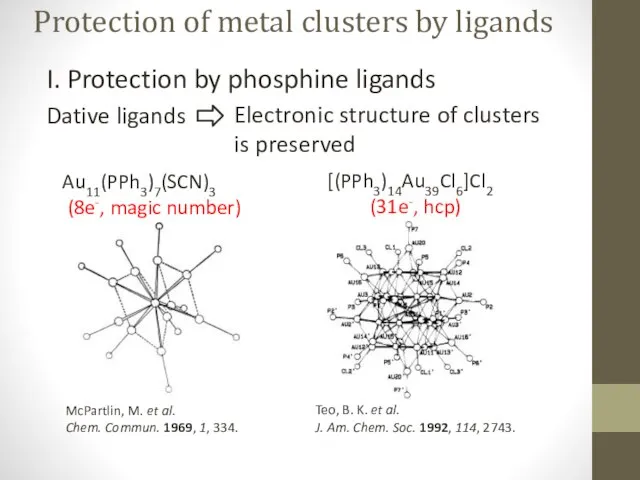
- 4. Protection of metal clusters by ligands I. Protection by phosphine ligands Dative ligands Au11(PPh3)7(SCN)3 (8e-, magic
- 5. Protection of metal clusters by ligands I. Protection by phosphine ligands Chiral clusters with BINAP [Au11(BINAP)4Br2]+
- 6. Protection of metal clusters by ligands II. Protection by thiolate ligands – high affinity between Au
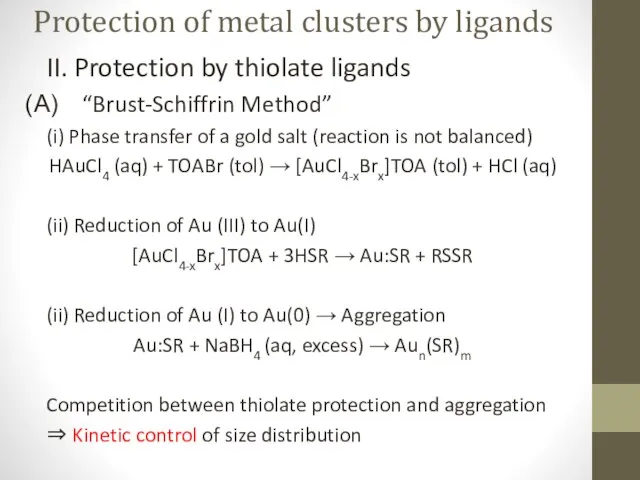
- 7. Protection of metal clusters by ligands II. Protection by thiolate ligands “Brust-Schiffrin Method” (i) Phase transfer
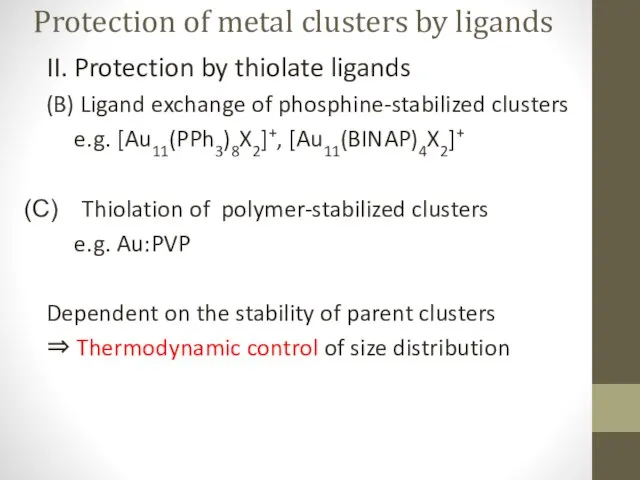
- 8. Protection of metal clusters by ligands II. Protection by thiolate ligands (B) Ligand exchange of phosphine-stabilized
- 9. Fractionation of gold clusters Atomic monodispersity is difficult to achieve ↓ Fractionation (A) Polyacrylamide gel electrophoresis
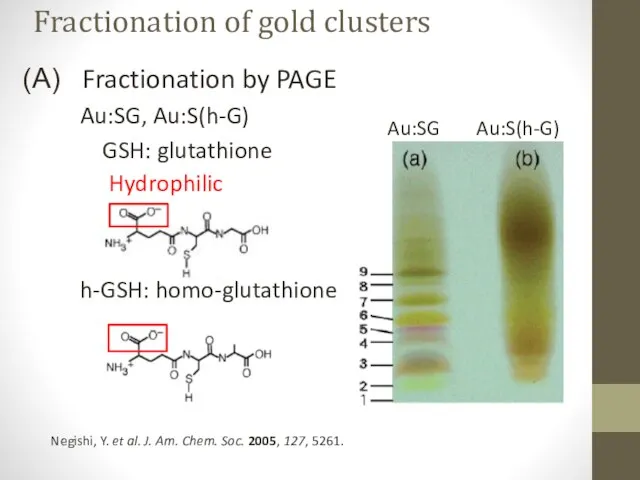
- 10. Fractionation by PAGE – suitable for hydrophilic, charged clusters Clusters with larger cores subject to stronger
- 11. Fractionation by PAGE Au:SG, Au:S(h-G) GSH: glutathione h-GSH: homo-glutathione Negishi, Y. et al. J. Am. Chem.
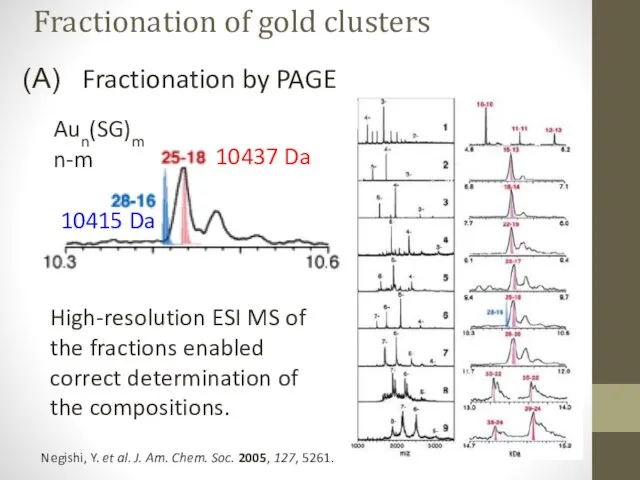
- 12. Fractionation by PAGE Negishi, Y. et al. J. Am. Chem. Soc. 2005, 127, 5261. High-resolution ESI
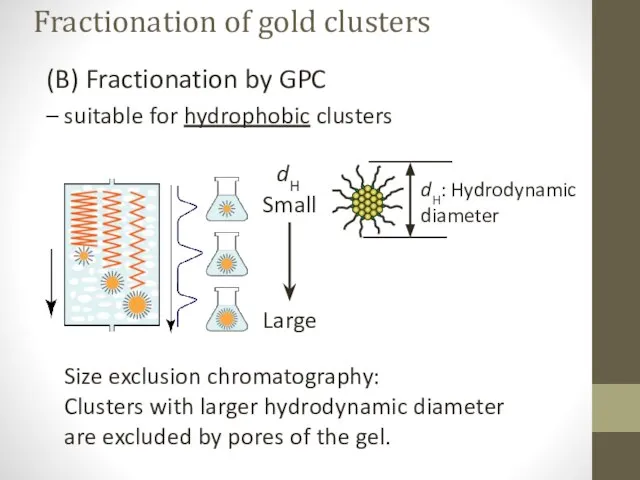
- 13. (B) Fractionation by GPC – suitable for hydrophobic clusters Size exclusion chromatography: Clusters with larger hydrodynamic
- 14. (B) Fractionation by GPC Tsunoyama, H. et al. J. Am. Chem. Soc. 2006, 128, 6036. Aun:SC18
- 15. (C) Size-selective etching Shichibu, Y. et al. Small 2007, 3, 835. “Size focusing” ― Thermodynamically stable
- 16. Stability of Au25(SG)18 Negishi, Y. et al. J. Am. Chem. Soc. 2007, 129, 11322. “Magic-numbered cluster”
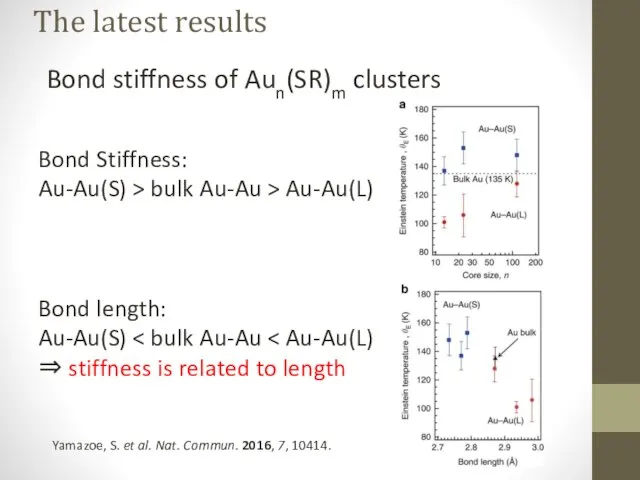
- 17. The latest results Bond stiffness of Aun(SR)m clusters Yamazoe, S. et al. Nat. Commun. 2016, 7,
- 18. The latest results Bond stiffness of Aun(SR)m clusters Bond Stiffness: Au-Au(S) > bulk Au-Au > Au-Au(L)
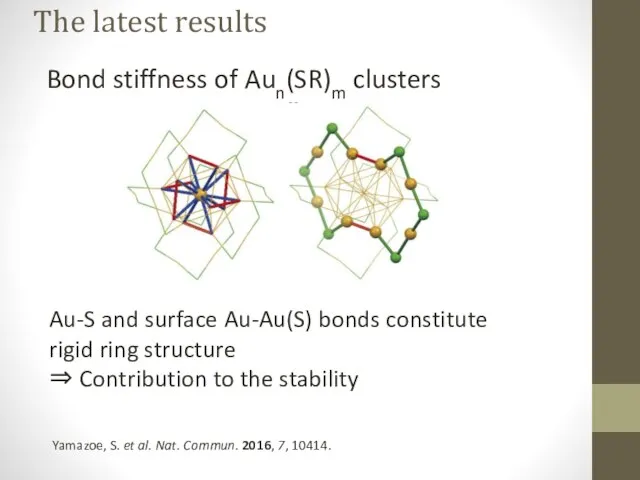
- 19. The latest results Bond stiffness of Aun(SR)m clusters Yamazoe, S. et al. Nat. Commun. 2016, 7,
- 20. Conclusion Bottom-up preparation of gold clusters can be achieved by protection with ligands. Monodisperse clusters are
- 22. Скачать презентацию









 Окислительно-восстановительное равновесие
Окислительно-восстановительное равновесие Материаловедение. Адсорбционные материалы. Металлоорганические каркасы
Материаловедение. Адсорбционные материалы. Металлоорганические каркасы Липиды. Общая характеристика липидов
Липиды. Общая характеристика липидов Біохімічний рівень організації
Біохімічний рівень організації Акимовская ООШ №1 приветствует учителей ХИМИИ района Выступление учителя химии Галак Р.С.
Акимовская ООШ №1 приветствует учителей ХИМИИ района Выступление учителя химии Галак Р.С. Новые требования, предъявляемые к лабораторно-минералогическим исследованиям
Новые требования, предъявляемые к лабораторно-минералогическим исследованиям 1. История и актуальность темы. 2. Получение стекломассы 3. Стеклянные изделия 4. Применение и виды стекла: Энергосберегающ
1. История и актуальность темы. 2. Получение стекломассы 3. Стеклянные изделия 4. Применение и виды стекла: Энергосберегающ Производные морфинана (фенантренизохинолина)
Производные морфинана (фенантренизохинолина) Стан, роль і місце органічної хімії в сучасній хімічній освіті.
Стан, роль і місце органічної хімії в сучасній хімічній освіті.  Химия и производство
Химия и производство Катализ. Лекция 1
Катализ. Лекция 1 Влияние на скорость ферментативной реакции
Влияние на скорость ферментативной реакции Поливинилхлорид (ПВХ, PVC)
Поливинилхлорид (ПВХ, PVC) Липиды. Общая характеристика и классификация. (Модуль 4.8)
Липиды. Общая характеристика и классификация. (Модуль 4.8) Производство бензина
Производство бензина Подготовка учащихся к практическим турам олимпиад по химии
Подготовка учащихся к практическим турам олимпиад по химии Кроссворд в картинках. Знакомство с формами и их элементами
Кроссворд в картинках. Знакомство с формами и их элементами Презентация по Химии "Синтетические моющие средства" - скачать смотреть
Презентация по Химии "Синтетические моющие средства" - скачать смотреть  Общая химическая технология
Общая химическая технология Анализ качества питьевой воды
Анализ качества питьевой воды  Реакции замещения и реакции обмена
Реакции замещения и реакции обмена Хлоридна кислота
Хлоридна кислота Физические свойства
Физические свойства Соединения железа
Соединения железа Химическая связь и ее виды
Химическая связь и ее виды История одного металла. Медь и её сплавы.
История одного металла. Медь и её сплавы.  Ֆլավանոիդներ պարունակող դեղաբույսեր եվ հումք
Ֆլավանոիդներ պարունակող դեղաբույսեր եվ հումք Классификация дисперсных систем. Коллигативные свойства растворов. Растворимость газов в воде. Термодинамика
Классификация дисперсных систем. Коллигативные свойства растворов. Растворимость газов в воде. Термодинамика