Содержание
- 2. Medical pharmacology is the science of chemicals (drugs) that interact with the human body. These interactions
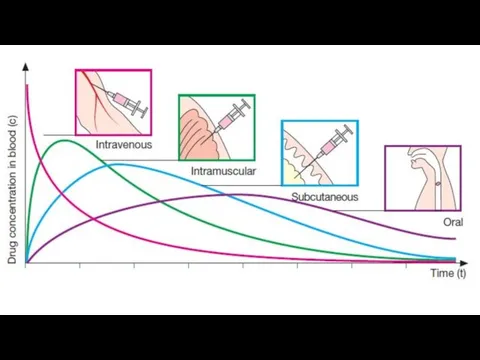
- 3. Routes of administration: ADVANTAGES and DISADVANTAGES Oral: the most common and safest, convenient, and economical route
- 5. Rectal: partially bypasses first-pass effect, destruction by stomach acid, ideal if drug causes vomiting, in patients
- 6. Intravenous: can have immediate effects, ideal if dosed in large volumes, suitable for irritating substances and
- 7. Subcutaneous: suitable for slow-release drugs, ideal for some poorly soluble suspensions, but pain or necrosis if
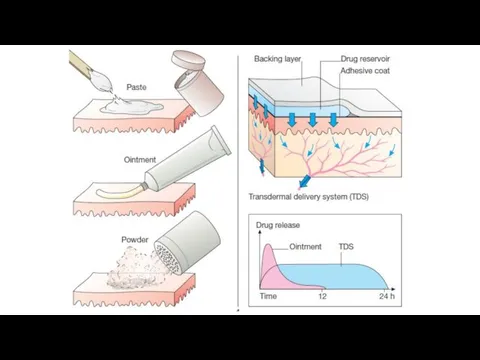
- 9. Transdermal (patch): bypasses the first-pass effect, convenient and painless, ideal for drugs that are lipophilic and
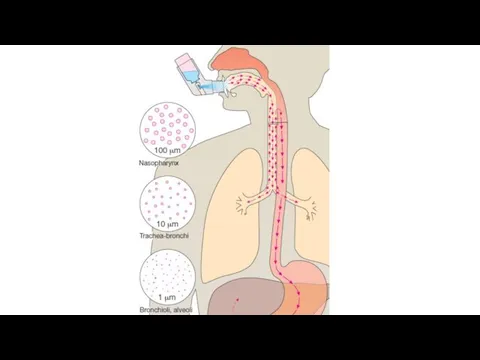
- 11. Inhalation: absorption is rapid; can have immediate effects, ideal for gases, effective for patients with respiratory
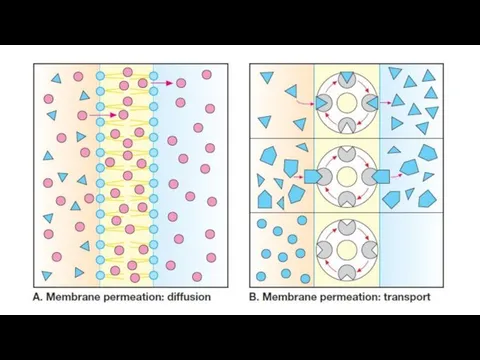
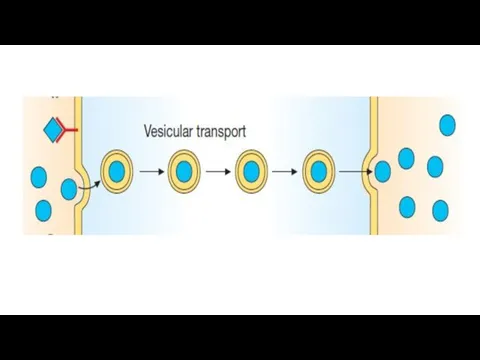
- 13. Mechanisms of absorption of drugs Drugs may be absorbed from the GI tract by passive diffusion,
- 14. Diffusion: lipid-soluble drugs readily move across most biologic membranes due to their solubility in the membrane
- 15. Active transport: Energy-dependent, involves specific carrier proteins. It is capable of moving drugs against a concentration
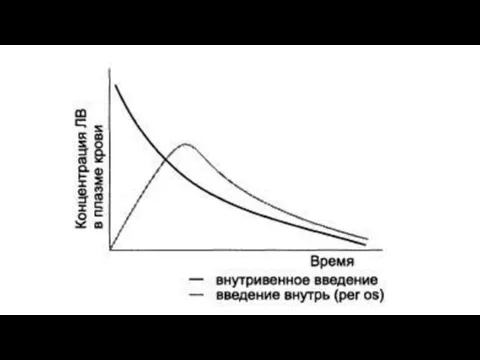
- 18. Bioavailability Bioavailability is the rate and extent to which an administered drug reaches the systemic circulation.
- 19. Determination of bioavailability: Bioavailability is determined by comparing plasma levels of a drug after a particular
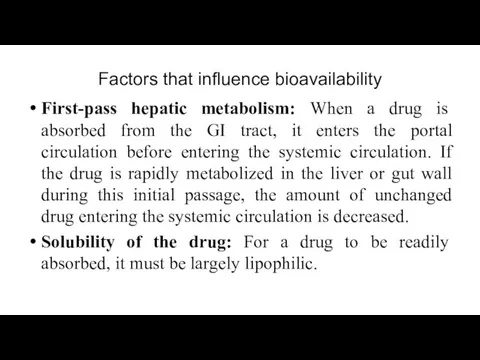
- 21. Factors that influence bioavailability First-pass hepatic metabolism: When a drug is absorbed from the GI tract,
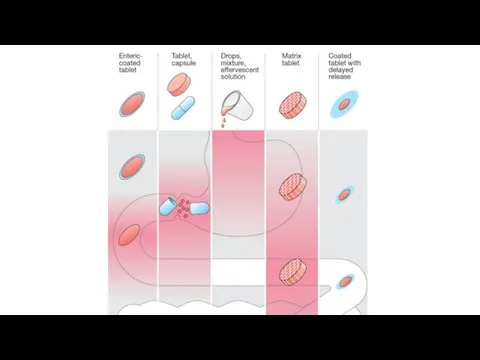
- 22. Chemical instability. Nature of the drug formulation (salt form, crystal polymorphism, enteric coatings, and the presence
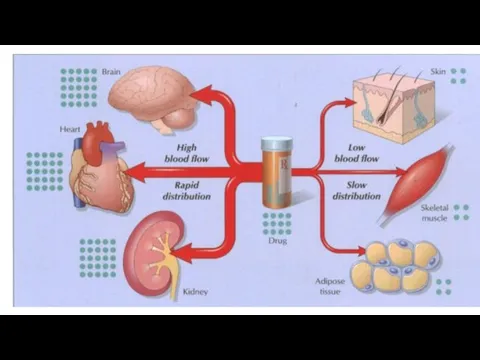
- 23. Drug distribution Drug distribution is the process by which a drug reversibly leaves the bloodstream and
- 24. Lipid-soluble drugs readily penetrate the CNS because they dissolve in the endothelial cell membrane. Ionized or
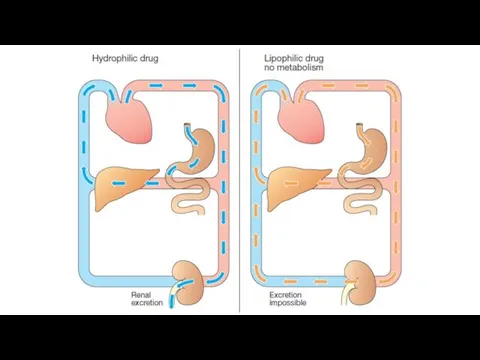
- 27. Elimination Once a drug enters the body, the process of elimination begins. The three major routes
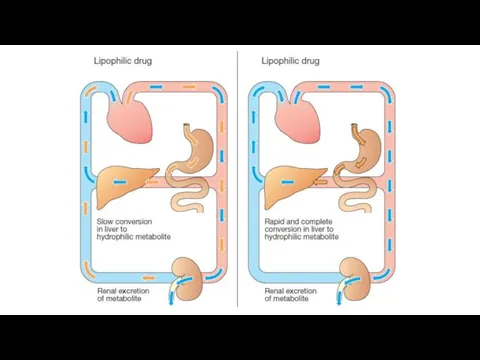
- 29. Metabolism in the liver leads to production of products with increased polarity, which allows the drug
- 31. The CYP450-dependent enzymes are an important target for pharmacokinetic drug interactions. Inducers (for example, phenobarbital, rifampin,
- 32. Phase II: This phase consists of conjugation reactions with an endogenous substrate, such as glucuronic acid,
- 33. Drug clearance may also occur via the intestines, bile, lungs, and breast milk, among others. Drugs
- 34. Pharmacodynamics. Effects Local effect occurs at the site of drug’s application . Resorptive (systemic) effect develops
- 35. Drugs “’targets Few drugs (e.g. activated charcoal, osmotic diuretics) act by virtue of their physicochemical properties,
- 36. Enzymes. Drugs that act by inhibiting enzymes include: anticholinesterases, which enhance the action of acetylcholine; carbonic
- 37. Drugs can influence on ion channels (selective pores in the membrane). Among drugs affecting ion channels
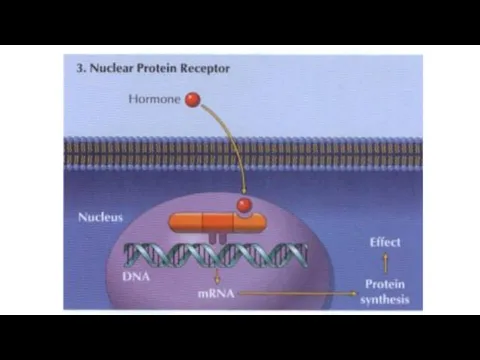
- 38. However, most drugs produce their effects by acting on specific proteins. These proteins are called receptors,
- 39. A receptor as any biologic molecule to which a drug binds and produces a measurable response.
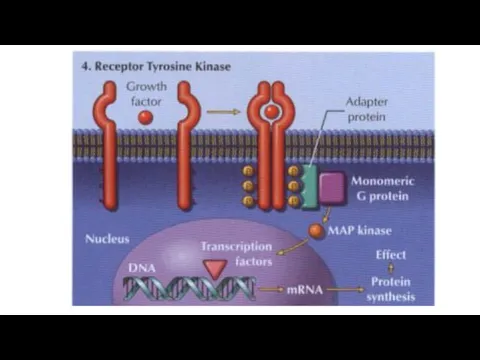
- 42. 3) Kinase‐linked receptors are surface receptors that possess (usually) intrinsic tyrosine kinase activity. They include receptors
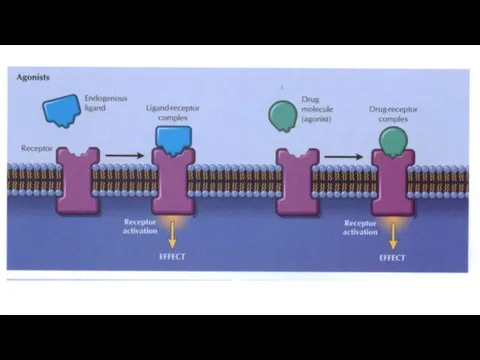
- 45. Chemicals (e.g. acetylcholine) or drugs that activate receptors and produce a response are called agonists .
- 46. Partial agonists. These are agonists that cannot elicit the same maximum response as a ‘full’ agonist.
- 49. The durability of the “Drug-receptor” bond determines whether the drug action is reversible (characteristic for most
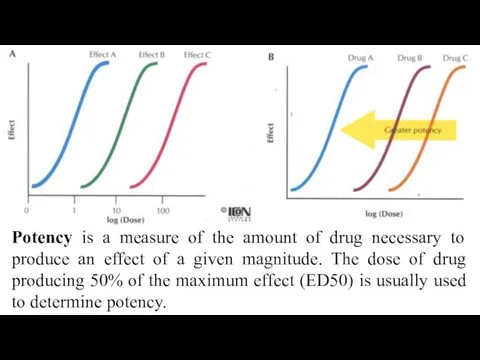
- 50. Potency is a measure of the amount of drug necessary to produce an effect of a
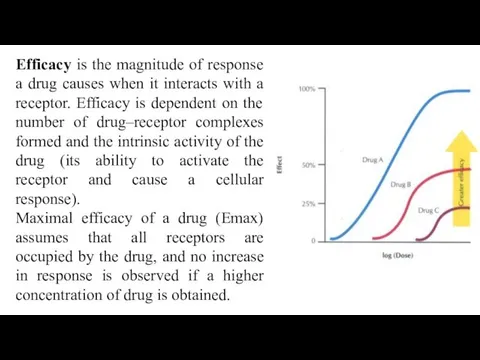
- 51. Efficacy is the magnitude of response a drug causes when it interacts with a receptor. Efficacy
- 52. Drugs interactions Pharmacokinetic Pharmacodynamic: Synergism Antagonism
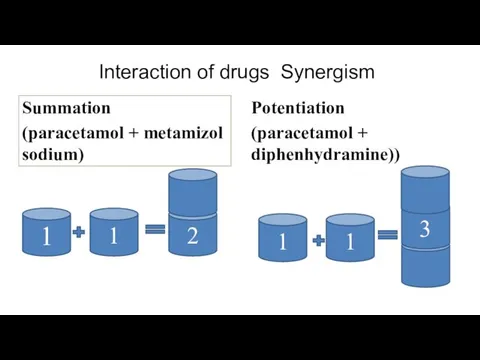
- 53. In case of synergism drug interaction leads to an increase in effect. Synergism may be direct
- 54. Interaction of drugs Synergism Summation (paracetamol + metamizol sodium) Potentiation (paracetamol + diphenhydramine)) 1 1 2
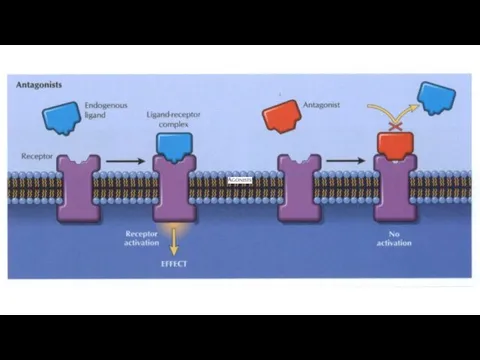
- 55. Antagonism The ability of a drug to decrease the effect of the other one is called
- 56. Irreversible antagonists have an effect that cannot be reversed by increasing the concentration of agonist. The
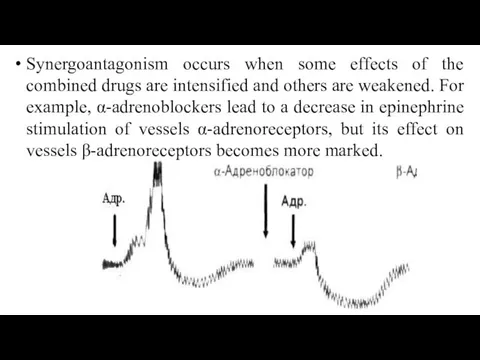
- 57. Synergoantagonism occurs when some effects of the combined drugs are intensified and others are weakened. For
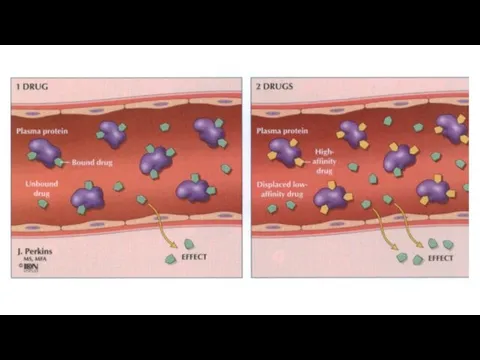
- 58. Pharmacokinetic interactions Absorption. Drugs that increase (e.g. metoclopramide) or decrease (e.g. atropine) the rate of gastric
- 59. Metabolism. Induction of hepatic enzymes by a second drug (e.g.phenobarbital, rifampicin) can decrease the efficacy of
- 60. Adverse drug reactions Adverse drug reactions can be divided into those that are predictable and dose‐related
- 61. Cumulation – storage of pharmacological substance in the body. It is typical for slow-acting drugs, that
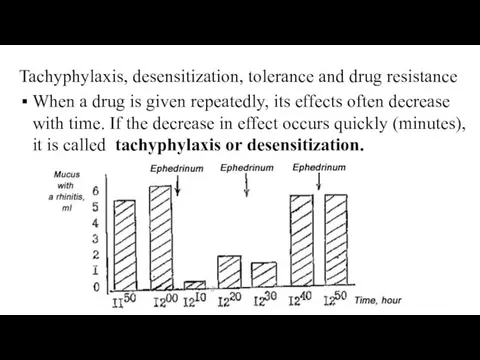
- 62. Tachyphylaxis, desensitization, tolerance and drug resistance When a drug is given repeatedly, its effects often decrease
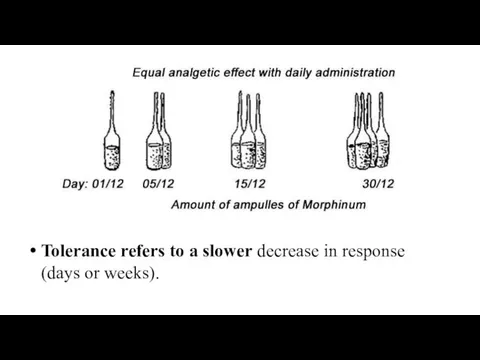
- 63. Tolerance refers to a slower decrease in response (days or weeks).
- 64. Tolerance may involve increased metabolism of a drug, e.g. ethanol, barbiturates, or homeostatic mechanisms (usually not
- 65. Drug dependence is a term used when a person has a compulsion to take a drug
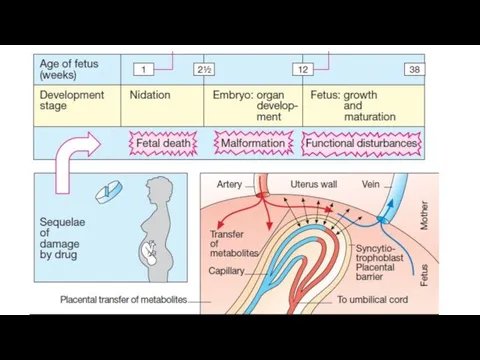
- 66. Teratogenesis is the occurrence of fetal developmental abnormalities caused by drugs taken during the first trimester
- 68. Carcinogenesis. Drug‐induced tumours are probably very rare because the pharmaceutical industry makes great efforts to avoid
- 70. Скачать презентацию











































 Сравнительная характеристика состояния полости рта и распространенности заболеваний кариеса между студентами 1-3 курса
Сравнительная характеристика состояния полости рта и распространенности заболеваний кариеса между студентами 1-3 курса Травмы зубов у детей
Травмы зубов у детей Целеполагание в поисковой деятельности
Целеполагание в поисковой деятельности Структура і принципи функціонування імунної системи. Імунологічні методи досліджень. Поняття про імунограму
Структура і принципи функціонування імунної системи. Імунологічні методи досліджень. Поняття про імунограму Абдоминальная хирургия
Абдоминальная хирургия Ультразвуковая (УЗ) терапия
Ультразвуковая (УЗ) терапия Рак яичников
Рак яичников Внимание. Оценка внимания младшего школьника
Внимание. Оценка внимания младшего школьника Инфекционный мононуклеоз
Инфекционный мононуклеоз Здоровый образ жизни и его составляющие
Здоровый образ жизни и его составляющие СДВГ у детей. Неустойчивость, отвлекаемость внимания. Импульсивность. Гиперактивность
СДВГ у детей. Неустойчивость, отвлекаемость внимания. Импульсивность. Гиперактивность Трансформационная игра «Путь к мечте»
Трансформационная игра «Путь к мечте» Межличностные отношения
Межличностные отношения Виды ран и общие правила оказания первой медицинской помощи
Виды ран и общие правила оказания первой медицинской помощи Нейропсихологическое обследование пациентов с деменцией
Нейропсихологическое обследование пациентов с деменцией Коммуникативные свойства личности в теории деятельности
Коммуникативные свойства личности в теории деятельности Other Psychotic Disorders
Other Psychotic Disorders Лучевые реакции и лучевые повреждения
Лучевые реакции и лучевые повреждения Введение в диетологию. Диетотерапия при нарушениях липидного обмена
Введение в диетологию. Диетотерапия при нарушениях липидного обмена Особенности профессиональной деятельности медицинской сестры в профилактике респираторных инфекций
Особенности профессиональной деятельности медицинской сестры в профилактике респираторных инфекций Нагноительные заболевания легких
Нагноительные заболевания легких Американская классическая школа психологии.Теория Г. Эмерсона
Американская классическая школа психологии.Теория Г. Эмерсона Ведение больных после эмболизации маточных сосудов
Ведение больных после эмболизации маточных сосудов Курация рожениц и родильниц
Курация рожениц и родильниц Роль информатизации в системе работы фельдшера медицинской организации
Роль информатизации в системе работы фельдшера медицинской организации Клиническая фармакология обезболевающих лекарственных средств
Клиническая фармакология обезболевающих лекарственных средств Создание композитной накладки своими руками
Создание композитной накладки своими руками Микоплазмоз
Микоплазмоз